Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation and immediate invasive assessment in refractory out-of-hospital cardiac arrest: a long-term follow-up of the Prague OHCA trial
- PMID: 38627823
- PMCID: PMC11022382
- DOI: 10.1186/s13054-024-04901-7
Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation and immediate invasive assessment in refractory out-of-hospital cardiac arrest: a long-term follow-up of the Prague OHCA trial
Abstract
Background: Randomized data evaluating the impact of the extracorporeal cardiopulmonary resuscitation (ECPR) approach on long-term clinical outcomes in patients with refractory out-of-hospital cardiac arrest (OHCA) are lacking. The objective of this follow-up study was to assess the long-term clinical outcomes of the ECPR-based versus CCPR approach.
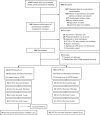
Methods: The Prague OHCA trial was a single-center, randomized, open-label trial. Patients with witnessed refractory OHCA of presumed cardiac origin, without return of spontaneous circulation, were randomized during ongoing resuscitation on scene to conventional CPR (CCPR) or an ECPR-based approach (intra-arrest transport, ECPR if ROSC is not achieved prehospital and immediate invasive assessment).
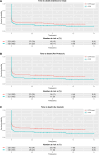
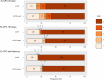
Results: From March 2013 to October 2020, 264 patients were randomized during ongoing resuscitation on scene, and 256 patients were enrolled. Long-term follow-up was performed 5.3 (interquartile range 3.8-7.2) years after initial randomization and was completed in 255 of 256 patients (99.6%). In total, 34/123 (27.6%) patients in the ECPR-based group and 26/132 (19.7%) in the CCPR group were alive (log-rank P = 0.01). There were no significant differences between the treatment groups in the neurological outcome, survival after hospital discharge, risk of hospitalization, major cardiovascular events and quality of life. Of long-term survivors, 1/34 (2.9%) in the ECPR-based arm and 1/26 (3.8%) in the CCPR arm had poor neurological outcome (both patients had a cerebral performance category score of 3).
Conclusions: Among patients with refractory OHCA, the ECPR-based approach significantly improved long-term survival. There were no differences in the neurological outcome, major cardiovascular events and quality of life between the groups, but the trial was possibly underpowered to detect a clinically relevant difference in these outcomes. Trial registration ClinicalTrials.gov Identifier: NCT01511666, Registered 19 January 2012.
Keywords: Extracorporeal cardiopulmonary resuscitation; Extracorporeal membrane oxygenation; Long-term; Out-of-hospital cardiac arrest; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
The corresponding author (JB) has received lecture honoraria from the Abiomed, Getinge, Xenios, Resuscitec, Novartis, Astra-Zeneca, Boegringer-Ingelheim. DR has received lecture honoraria from the Abiomed and Resuscitec. The remaining authors report no conflict of interest.
Figures
References
-
- Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327(8):737–747. doi: 10.1001/jama.2022.1025. - DOI - PMC - PubMed
-
- Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807–1816. doi: 10.1016/S0140-6736(20)32338-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- MH CZ - DRO - VFN00064165/Ministerstvo Zdravotnictví Ceské Republiky
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
- Cooperatio - Intensive Care Medicine/Univerzita Karlova v Praze
LinkOut - more resources
Full Text Sources
Medical

