Gut microbiota-host lipid crosstalk in Alzheimer's disease: implications for disease progression and therapeutics
- PMID: 38627829
- PMCID: PMC11020986
- DOI: 10.1186/s13024-024-00720-0
Gut microbiota-host lipid crosstalk in Alzheimer's disease: implications for disease progression and therapeutics
Abstract
Trillions of intestinal bacteria in the human body undergo dynamic transformations in response to physiological and pathological changes. Alterations in their composition and metabolites collectively contribute to the progression of Alzheimer's disease. The role of gut microbiota in Alzheimer's disease is diverse and complex, evidence suggests lipid metabolism may be one of the potential pathways. However, the mechanisms that gut microbiota mediate lipid metabolism in Alzheimer's disease pathology remain unclear, necessitating further investigation for clarification. This review highlights the current understanding of how gut microbiota disrupts lipid metabolism and discusses the implications of these discoveries in guiding strategies for the prevention or treatment of Alzheimer's disease based on existing data.
Keywords: APOE; Alzheimer’s disease; Cholesterol; Exercise; Gut microbiota; LPS; Lifestyle; Lipid metabolism; Neuroinflammation; Probiotics; SCFAs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
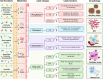
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- No.82371427/National Natural Science Foundation of China
- CSTB2023NSCQBHX0018/Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission
- CSTB2023NSCQ-MSX0323/Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission
- No. kryc-lj-2105/Chongqing Medical University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

