The impact of within-host coinfection interactions on between-host parasite transmission dynamics varies with spatial scale
- PMID: 38628126
- PMCID: PMC11021925
- DOI: 10.1098/rspb.2024.0103
The impact of within-host coinfection interactions on between-host parasite transmission dynamics varies with spatial scale
Abstract
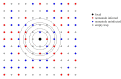
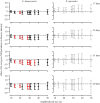
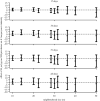
Within-host interactions among coinfecting parasites can have major consequences for individual infection risk and disease severity. However, the impact of these within-host interactions on between-host parasite transmission, and the spatial scales over which they occur, remain unknown. We developed and apply a novel spatially explicit analysis to parasite infection data from a wild wood mouse (Apodemus sylvaticus) population. We previously demonstrated a strong within-host negative interaction between two wood mouse gastrointestinal parasites, the nematode Heligmosomoides polygyrus and the coccidian Eimeria hungaryensis, using drug-treatment experiments. Here, we show this negative within-host interaction can significantly alter the between-host transmission dynamics of E. hungaryensis, but only within spatially restricted neighbourhoods around each host. However, for the closely related species E. apionodes, which experiments show does not interact strongly with H. polygyrus, we did not find any effect on transmission over any spatial scale. Our results demonstrate that the effects of within-host coinfection interactions can ripple out beyond each host to alter the transmission dynamics of the parasites, but only over local scales that likely reflect the spatial dimension of transmission. Hence there may be knock-on consequences of drug treatments impacting the transmission of non-target parasites, altering infection risks even for non-treated individuals in the wider neighbourhood.
Keywords: coinfection; disease ecology; disease spread; parasite ecology; parasite interactions; spatial ecology.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
