Rapid Enrichment of a Native Multipass Transmembrane Protein via Cell Membrane Electrophoresis through Buffer pH and Ionic Strength Adjustment
- PMID: 38628144
- PMCID: PMC11066866
- DOI: 10.1021/jacs.3c13579
Rapid Enrichment of a Native Multipass Transmembrane Protein via Cell Membrane Electrophoresis through Buffer pH and Ionic Strength Adjustment
Abstract
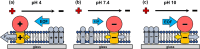
Supported membrane electrophoresis is a promising technique for collecting membrane proteins in native bilayer environments. However, the slow mobility of typical transmembrane proteins has impeded the technique's advancement. Here, we successfully applied cell membrane electrophoresis to rapidly enrich a 12-transmembrane helix protein, glucose transporter 1 with antibodies (GLUT1 complex), by tuning the buffer pH and ionic strength. The identified conditions allowed the separation of the GLUT1 complex and a lipid probe, Fast-DiO, within a native-like environment in a few minutes. A force model was developed to account for distinct electric and drag forces acting on the transmembrane and aqueous-exposed portion of a transmembrane protein as well as the electroosmotic force. This model not only elucidates the impact of size and charge properties of transmembrane proteins but also highlights the influence of pH and ionic strength on the driving forces and, consequently, electrophoretic mobility. Model predictions align well with experimentally measured electrophoretic mobilities of the GLUT1 complex and Fast-DiO at various pH and ionic strengths as well as with several lipid probes, lipid-anchored proteins, and reconstituted membrane proteins from previous studies. Force analyses revealed the substantial membrane drag of the GLUT1 complex, significantly slowing down electrophoretic mobility. Besides, the counterbalance of similar magnitudes of electroosmotic and electric forces results in a small net driving force and, consequently, reduced mobility under typical neutral pH conditions. Our results further highlight how the size and charge properties of transmembrane proteins influence the suitable range of operating conditions for effective movement, providing potential applications for concentrating and isolating membrane proteins within this platform.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Supported bilayer electrophoresis under controlled buffer conditions.Anal Chem. 2011 Mar 15;83(6):2090-6. doi: 10.1021/ac1028819. Epub 2011 Feb 14. Anal Chem. 2011. PMID: 21319743 Free PMC article.
-
Correlation of electrophoretic mobilities of proteins and peptides with their physicochemical properties.Anal Biochem. 1995 Mar 20;226(1):51-8. doi: 10.1006/abio.1995.1190. Anal Biochem. 1995. PMID: 7785779
-
Determination of electroosmotic and electrophoretic mobility of DNA and dyes in low ionic strength solutions.Electrophoresis. 2018 Mar;39(5-6):862-868. doi: 10.1002/elps.201700281. Epub 2017 Sep 15. Electrophoresis. 2018. PMID: 28834563
-
Comparative study of analytical techniques for determining protein charge.J Pharm Sci. 2015 Jul;104(7):2123-31. doi: 10.1002/jps.24454. Epub 2015 Apr 24. J Pharm Sci. 2015. PMID: 25911989 Review.
-
History and principles of conductive media for standard DNA electrophoresis.Anal Biochem. 2004 Oct 1;333(1):1-13. doi: 10.1016/j.ab.2004.05.054. Anal Biochem. 2004. PMID: 15351274 Review.
Cited by
-
Interpretable Multiscale Convolutional Neural Network for Classification and Feature Visualization of Weak Raman Spectra of Biomolecules at Cell Membranes.ACS Sens. 2025 Apr 25;10(4):2652-2666. doi: 10.1021/acssensors.4c03260. Epub 2025 Apr 4. ACS Sens. 2025. PMID: 40184533 Free PMC article.
References
-
- Yang Z.; Wang C.; Zhou Q.; An J.; Hildebrandt E.; Aleksandrov L. A.; Kappes J. C.; DeLucas L. J.; Riordan J. R.; Urbatsch I. L.; et al. Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains. Protein Sci. 2014, 23 (6), 769–789. 10.1002/pro.2460. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

