Differential impact of fentanyl and morphine doses on ticagrelor-induced platelet inhibition in ST-segment elevation myocardial infarction: a subgroup analysis from the PERSEUS randomized trial
- PMID: 38628315
- PMCID: PMC11018886
- DOI: 10.3389/fcvm.2024.1324641
Differential impact of fentanyl and morphine doses on ticagrelor-induced platelet inhibition in ST-segment elevation myocardial infarction: a subgroup analysis from the PERSEUS randomized trial
Abstract
Introduction: Among patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI), intravenous fentanyl does not enhance ticagrelor-induced platelet inhibition within 2 h compared to morphine. The impact of the total dose of fentanyl and morphine received on ticagrelor pharmacodynamic and pharmacokinetic responses in patients with STEMI remains however undetermined.
Materials and methods: We performed a post-hoc subanalysis of the prospective, open-label, single-center, randomized PERSEUS trial (NCT02531165) that compared treatment with intravenous fentanyl vs. morphine among symptomatic patients with STEMI treated with primary PCI after ticagrelor pretreatment. Patients from the same population as PERSEUS were further stratified according to the total dose of intravenous opioids received. The primary outcome was platelet reactivity using P2Y12 reaction units (PRU) at 2 h following administration of a loading dose (LD) of ticagrelor. Secondary outcomes were platelet reactivity and peak plasma levels of ticagrelor and AR-C124910XX, its active metabolite, at up to 12 h after ticagrelor LD administration. Generalized linear models for repeated measures were built to determine the relationship between raw and weight-weighted doses of fentanyl and morphine.
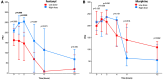
Results: 38 patients with STEMI were included between December 18, 2015, and June 22, 2017. Baseline clinical and procedural characteristics were similar between low- and high-dose opioid subgroups. At 2 h, there was a significant correlation between PRU and both raw [regression coefficient (B), 0.51; 95% confidence interval (CI), 0.02-0.99; p = 0.043] and weight-weighted (B, 0.54; 95% CI, 0.49-0.59; p < 0.001) doses of fentanyl, but not morphine. Median PRU at 2 h was significantly lower in patients receiving low, as compared to high, doses of fentanyl [147; interquartile range (IQR), 63-202; vs. 255; IQR, 183-274; p = 0.028], whereas no significant difference was found in those receiving morphine (217; IQR, 165-266; vs. 237; IQR, 165-269; p = 0.09). At 2 h, weight-weighted doses of fentanyl and morphine were significantly correlated to plasma levels of ticagrelor and AR-C124910XX.
Conclusion: In symptomatic patients with STEMI who underwent primary PCI after ticagrelor pretreatment and who received intravenous opioids, we found a dose-dependent relationship between the administration of intravenous fentanyl, but not morphine, and ticagrelor-induced platelet inhibition.
Keywords: ST-segment elevation myocardial infarction; dose; fentanyl; pharmacodynamics; pharmacokinetics; ticagrelor.
© 2024 Garin, Degrauwe, Carbone, Musayeb, Lauriers, Valgimigli and Iglesias.
Conflict of interest statement
JFI has disclosed receiving research funding for his institution (Lausanne University Hospital, Switzerland) and personal compensation from AstraZeneca during the study; institutional research grants and personal fees from Abbott Vascular, Biotronik, Biosensors, Concept Medical, Philips Volcano, Terumo Corporation, in addition to personal fees from AstraZeneca, Biotronik, Biosensors, Concept Medical, Terumo Corporation, Medtronic, Medalliance, Novartis, Pfizer, Bristol Myers Squibb, and Cordis, related to activities outside the submitted manuscript; MV has acknowledged receiving both grants and personal fees from Abbott, Terumo Corporation, and AstraZeneca, personal fees from Bayer, Daiichi Sankyo, Amgen, Alvimedica, Idorsia, Coreflow, Vifor, BristolMyers Squibb, and iVascular, and funding from Medicare, all concerning endeavors outside the submitted work; SD has reported obtaining grants and personal fees from Biotronik, also unrelated to the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Influence of intravenous fentanyl compared with morphine on ticagrelor absorption and platelet inhibition in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: rationale and design of the PERSEUS randomized trial.Eur Heart J Cardiovasc Pharmacother. 2019 Jul 1;5(3):158-163. doi: 10.1093/ehjcvp/pvy031. Eur Heart J Cardiovasc Pharmacother. 2019. PMID: 30101278
-
Comparative effects of fentanyl versus morphine on platelet inhibition induced by ticagrelor in patients with ST-segment elevation myocardial infarction: Full results of the PERSEUS randomized trial.Cardiol J. 2022;29(4):591-600. doi: 10.5603/CJ.a2022.0049. Epub 2022 Jun 28. Cardiol J. 2022. PMID: 35762079 Free PMC article. Clinical Trial.
-
Impact of Escalating Loading Dose Regimens of Ticagrelor in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of a Prospective Randomized Pharmacokinetic and Pharmacodynamic Investigation.JACC Cardiovasc Interv. 2015 Sep;8(11):1457-1467. doi: 10.1016/j.jcin.2015.02.030. JACC Cardiovasc Interv. 2015. PMID: 26404199 Clinical Trial.
-
Association of periprocedural intravenous morphine use on clinical outcomes in ST-elevation myocardial infarction (STEMI) treated by primary percutaneous coronary intervention: Systematic review and meta-analysis.Catheter Cardiovasc Interv. 2020 Jul;96(1):76-88. doi: 10.1002/ccd.28561. Epub 2019 Oct 26. Catheter Cardiovasc Interv. 2020. PMID: 31654491
-
Use of ticagrelor alongside fibrinolytic therapy in patients with ST-segment elevation myocardial infarction: Practical perspectives based on data from the TREAT study.Clin Cardiol. 2018 Oct;41(10):1322-1327. doi: 10.1002/clc.23043. Epub 2018 Oct 16. Clin Cardiol. 2018. PMID: 30098028 Free PMC article. Review.
Cited by
-
Opioids in Critically Ill Acute Myocardial Infarction Patients: A Retrospective Analysis of the MIMIC-IV Database.J Gen Intern Med. 2025 Jun 25. doi: 10.1007/s11606-025-09643-y. Online ahead of print. J Gen Intern Med. 2025. PMID: 40562883
-
Fentanyl and Sudden Death-A Postmortem Perspective for Diagnosing and Predicting Risk.Diagnostics (Basel). 2024 Sep 9;14(17):1995. doi: 10.3390/diagnostics14171995. Diagnostics (Basel). 2024. PMID: 39272779 Free PMC article. Review.
References
-
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44(38):3720–826. 10.1093/eurheartj/ehad191 - DOI - PubMed
-
- Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. (2012) 5(6):797–804. 10.1161/CIRCINTERVENTIONS.112.972323 - DOI - PubMed
-
- Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: rapid (rapid activity of platelet inhibitor drugs) primary PCI study. J Am Coll Cardiol. (2013) 61(15):1601–6. 10.1016/j.jacc.2013.01.024 - DOI - PubMed
-
- Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Sroka WD, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled impression trial. Eur Heart J. (2016) 37(3):245–52. 10.1093/eurheartj/ehv547 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

