Microbial dysbiosis in roots and rhizosphere of grapevines experiencing decline is associated with active metabolic functions
- PMID: 38628369
- PMCID: PMC11018932
- DOI: 10.3389/fpls.2024.1358213
Microbial dysbiosis in roots and rhizosphere of grapevines experiencing decline is associated with active metabolic functions
Abstract
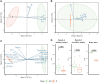
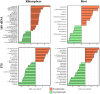
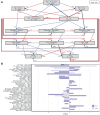
When grapevine decline, characterized by a premature decrease in vigor and yield and sometimes plant death, cannot be explained by pathological or physiological diseases, one may inquire whether the microbiological status of the soil is responsible. Previous studies have shown that the composition and structure of bacterial and fungal microbial communities in inter-row soil are affected in areas displaying vine decline, compared to areas with non-declining vines within the same plot. A more comprehensive analysis was conducted in one such plot. Although soil chemical parameters could not directly explain these differences, the declining vines presented lower vigor, yield, berry quality, and petiole mineral content than those in non-declining vines. The bacterial and fungal microbiome of the root endosphere, rhizosphere, and different horizons of the bulk soil were explored through enzymatic, metabolic diversity, and metabarcoding analysis in both areas. Despite the lower microbial diversity and richness in symptomatic roots and soil, higher microbial activity and enrichment of potentially both beneficial bacteria and pathogenic fungi were found in the declining area. Path modeling analysis linked the root microbial activity to berry quality, suggesting a determinant role of root microbiome in the berry mineral content. Furthermore, certain fungal and bacterial taxa were correlated with predicted metabolic pathways and metabolic processes assessed with Eco-Plates. These results unexpectedly revealed active microbial profiles in the belowground compartments associated with stressed vines, highlighting the interest of exploring the functional microbiota of plants, and more specifically roots and rhizosphere, under stressed conditions.
Keywords: Vitis vinifera growth; belowground microbiome; grapevine fitness; metabarcoding-based predicted functionality; root endophytes; soil quality.
Copyright © 2024 Darriaut, Marzari, Lailheugue, Tran, Martins, Marguerit, Masneuf-Pomarède and Lauvergeat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Soil composition and rootstock genotype drive the root associated microbial communities in young grapevines.Front Microbiol. 2022 Nov 10;13:1031064. doi: 10.3389/fmicb.2022.1031064. eCollection 2022. Front Microbiol. 2022. PMID: 36439844 Free PMC article.
-
Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality.Microbiome. 2018 Jan 3;6(1):3. doi: 10.1186/s40168-017-0391-2. Microbiome. 2018. PMID: 29298729 Free PMC article.
-
Epichloë Endophyte Infection Changes the Root Endosphere Microbial Community Composition of Leymus Chinensis Under Both Potted and Field Growth Conditions.Microb Ecol. 2023 Feb;85(2):604-616. doi: 10.1007/s00248-022-01983-0. Epub 2022 Feb 23. Microb Ecol. 2023. PMID: 35194659
-
Plant domestication shapes rhizosphere microbiome assembly and metabolic functions.Microbiome. 2023 Mar 31;11(1):70. doi: 10.1186/s40168-023-01513-1. Microbiome. 2023. PMID: 37004105 Free PMC article.
-
Roots shaping their microbiome: global hotspots for microbial activity.Annu Rev Phytopathol. 2015;53:403-24. doi: 10.1146/annurev-phyto-082712-102342. Annu Rev Phytopathol. 2015. PMID: 26243728 Review.
Cited by
-
Diversity and functional features of the root-associated bacteriome are dependent on grapevine susceptibility to Plasmopara viticola.Environ Microbiome. 2025 Mar 14;20(1):30. doi: 10.1186/s40793-025-00690-w. Environ Microbiome. 2025. PMID: 40087775 Free PMC article.
-
Rhizobacteria from vineyard and commercial arbuscular mycorrhizal fungi induce synergistic microbiome shifts within grapevine root systems.Sci Rep. 2025 Jul 30;15(1):27884. doi: 10.1038/s41598-025-12673-5. Sci Rep. 2025. PMID: 40739286 Free PMC article.
References
-
- Andrews S. (2010) FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
LinkOut - more resources
Full Text Sources

