Association analyses between the variants of SNAP25, SV2C and ST3GAL2 and the efficacy of botulinum toxin A in the treatment of the primary Meige syndrome
- PMID: 38628704
- PMCID: PMC11019161
- DOI: 10.1016/j.heliyon.2024.e28543
Association analyses between the variants of SNAP25, SV2C and ST3GAL2 and the efficacy of botulinum toxin A in the treatment of the primary Meige syndrome
Abstract
Objective: Individual differences were observed in the clinical efficacy of Botulinum toxin A (BoNT-A) in the treatment of the primary Meige syndrome. Our study aimed to explore the potential associations between the clinical efficacy of BoNT-A in the treatment of the primary Meige syndrome and variants of SNAP25, SV2C and ST3GAL2, which are involving in the translocation of the BoNT-A in vivo.
Methods: Patients with the primary Meige syndrome treated with BoNT-A were enrolled. Clinical efficacy was evaluated by the maximum improvement rate of motor symptoms and the duration of efficacy. Variants of SNAP25, SV2C and ST3GAL2 were obtained by Sanger sequencing. Another cohort diagnosed with primary cervical dystonia was also enrolled in the replication stage.
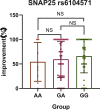
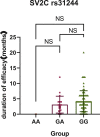
Results: Among the 104 primary Meige syndrome patients, 80 patients (76.9%) had a good efficacy (the maximum improvement rate of motor symptoms ≥30%) and 24 (23. 1%) had a poor (the maximum improvement rate of motor symptoms <30%). As to the duration of efficacy, 52 patients (50.0%) had a long duration of efficacy (≥4 months), and 52 (50.0%) had a short (<4 months). In terms of primary Meige syndrome, SNAP25 rs6104571 was found associating with the maximum improvement rate of motor symptoms (Genotype: P = 0.02, OR = 0.26; Allele: P = 0.013, OR = 0.29), and SV2C rs31244 was found associating with the duration of efficacy (Genotype: P = 0.024, OR = 0.13; Allele: P = 0.012, OR = 0.13). Besides, we also conducted the association analyses between the variants and BoNT-A-related adverse reactions. Although, there was no statistical difference between the allele of SV2C rs31244 and BoNT-A-related adverse reactions, there was a trend (P = 0.077, OR = 2.56). In the replication stage, we included 39 patients with primary cervical dystonia to further expanding the samples' size. Among the 39 primary cervical dystonia patients, 25 patients (64.1%) had a good efficacy (the maximum improvement rate of motor symptoms ≥50%) and 14 (35.9%) had a poor (the maximum improvement rate of motor symptoms <50%). As to the duration of efficacy, 32 patients (82.1%) had a long duration of efficacy (≥6 months), and 7 (17.9%) had a short (<6 months). Integrating primary Meige syndrome and primary cervical dystonia, SV2C rs31244 was still found associating with the duration of efficacy (Genotype: P = 0.002, OR = 0. 23; Allele: P = 0.001, OR = 0. 25).
Conclusion: In our study, SNAP25 rs6104571 was associated with the maximum improvement rate of motor symptoms in patients with primary Meige syndrome treated with BoNT-A, and patients carrying this variant had a lower improvement rate of motor symptoms. SV2C rs31244 was associated with duration of treatment in patients with primary Meige syndrome treated with BoNT-A and patients carrying this variant had a shorter duration of treatment. Patients with primary Meige syndrome carrying SV2C rs31244 G allele have an increase likelihood of BoNT-A-related adverse reactions. Involving 39 patients with primary cervical dystonia, the results further verify that SV2C rs31244 was associated with duration of treatment and patients carrying this variant had a shorter duration of treatment.
Keywords: Botulinum toxin A; Cervical dystonia; Meige syndrome; SNAP25; ST3GAL2; SV2C.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Long-Term Efficacy of Deep Brain Stimulation of Bilateral Globus Pallidus Internus in Primary Meige Syndrome.Stereotact Funct Neurosurg. 2019;97(5-6):356-361. doi: 10.1159/000504861. Epub 2020 Jan 10. Stereotact Funct Neurosurg. 2019. PMID: 31927550
-
Botulinum Neurotoxin A4 Has a 1000-Fold Reduced Potency Due to Three Single Amino Acid Alterations in the Protein Receptor Binding Domain.Int J Mol Sci. 2023 Mar 16;24(6):5690. doi: 10.3390/ijms24065690. Int J Mol Sci. 2023. PMID: 36982762 Free PMC article.
-
Efficacy and safety of long-term botulinum toxin treatment for acquired cervical dystonia: a 25-year follow-up.J Neurol. 2023 Jan;270(1):340-347. doi: 10.1007/s00415-022-11343-0. Epub 2022 Sep 6. J Neurol. 2023. PMID: 36068376 Free PMC article.
-
Deep Brain Stimulation for Focal or Segmental Craniocervical Dystonia in Patients Who Have Failed Botulinum Neurotoxin Therapy-A Narrative Review of the Literature.Toxins (Basel). 2023 Oct 9;15(10):606. doi: 10.3390/toxins15100606. Toxins (Basel). 2023. PMID: 37888637 Free PMC article. Review.
-
Long-term efficacy and safety of botulinum toxin treatment for cervical dystonia: a critical reappraisal.Expert Opin Drug Saf. 2021 Jun;20(6):695-705. doi: 10.1080/14740338.2021.1915282. Epub 2021 Jun 28. Expert Opin Drug Saf. 2021. PMID: 33831328 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials