Discovery of α-Amidobenzylboronates as Highly Potent Covalent Inhibitors of Plasma Kallikrein
- PMID: 38628785
- PMCID: PMC11017388
- DOI: 10.1021/acsmedchemlett.3c00572
Discovery of α-Amidobenzylboronates as Highly Potent Covalent Inhibitors of Plasma Kallikrein
Abstract
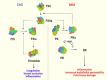
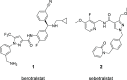
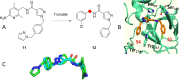
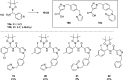
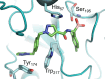
Hereditary angioedema (HAE), a rare genetic disorder, is associated with uncontrolled plasma kallikrein (PKa) enzyme activity leading to the generation of bradykinin swelling in subcutaneous and submucosal membranes in various locations of the body. Herein, we describe a series of potent α-amidobenzylboronates as potential covalent inhibitors of PKa. These compounds exhibited time-dependent inhibition of PKa (compound 20 IC50 66 nM at 1 min, 70 pM at 24 h). Further compound dissociation studies demonstrated that 20 showed no apparent reversibility comparable to d-Phe-Pro-Arg-chloromethylketone (PPACK) (23), a known nonselective covalent PKa inhibitor.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Sebetralstat (KVD900): A Potent and Selective Small Molecule Plasma Kallikrein Inhibitor Featuring a Novel P1 Group as a Potential Oral On-Demand Treatment for Hereditary Angioedema.J Med Chem. 2022 Oct 27;65(20):13629-13644. doi: 10.1021/acs.jmedchem.2c00921. Epub 2022 Oct 17. J Med Chem. 2022. PMID: 36251573 Free PMC article.
-
Specific Targeting of Plasma Kallikrein for Treatment of Hereditary Angioedema: A Revolutionary Decade.J Allergy Clin Immunol Pract. 2022 Mar;10(3):716-722. doi: 10.1016/j.jaip.2021.11.011. Epub 2021 Nov 25. J Allergy Clin Immunol Pract. 2022. PMID: 34838707
-
Measurement of Bradykinin Formation and Degradation in Blood Plasma: Relevance for Acquired Angioedema Associated With Angiotensin Converting Enzyme Inhibition and for Hereditary Angioedema Due to Factor XII or Plasminogen Gene Variants.Front Med (Lausanne). 2020 Jul 17;7:358. doi: 10.3389/fmed.2020.00358. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32766265 Free PMC article.
-
Hereditary and acquired C1-inhibitor-dependent angioedema: from pathophysiology to treatment.Ann Med. 2016;48(4):256-67. doi: 10.3109/07853890.2016.1162909. Epub 2016 Mar 26. Ann Med. 2016. PMID: 27018196 Review.
-
Bradykinin-mediated diseases.Chem Immunol Allergy. 2014;100:140-7. doi: 10.1159/000358619. Epub 2014 May 22. Chem Immunol Allergy. 2014. PMID: 24925394 Review.
References
-
- Li C.; Barroeta A. B.; Wong S. S.; Kim H. J.; Pathak M.; Dreveny I.; Meijers J. C. M.; Emsley J. Structures of Factor XI and Prekallikrein Bound to Domain 6 of High–Molecular Weight Kininogen Reveal Alternate Domain 6 Conformations and Exosites. Journal of Thrombosis and Haemostasis 2023, 21 (9), 2378–2389. 10.1016/j.jtha.2023.03.042. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information

