Discovery of Hepatitis B Virus Surface Antigen Suppressor GS-8873
- PMID: 38628802
- PMCID: PMC11017420
- DOI: 10.1021/acsmedchemlett.4c00037
Discovery of Hepatitis B Virus Surface Antigen Suppressor GS-8873
Abstract
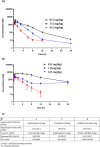
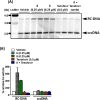
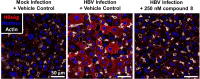
Chronic hepatitis B (CHB) virus infection afflicts hundreds of millions of people and causes nearly one million deaths annually. The high levels of circulating viral surface antigen (HBsAg) that characterize CHB may lead to T-cell exhaustion, resulting in an impaired antiviral immune response in the host. Agents that suppress HBsAg could help invigorate immunity toward infected hepatocytes and facilitate a functional cure. A series of dihydropyridoisoquinolizinone (DHQ) inhibitors of human poly(A) polymerases PAPD5/7 were reported to suppress HBsAg in vitro. An example from this class, RG7834, briefly entered the clinic. We set out to identify a potent, orally bioavailable, and safe PAPD5/7 inhibitor as a potential component of a functional cure regimen. Our efforts led to the identification of a dihydropyridophthalazinone (DPP) core with improved pharmacokinetic properties. A conformational restriction strategy and optimization of core substitution led to GS-8873, which was projected to provide deep HBsAg suppression with once-daily dosing.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
A novel orally available small molecule that inhibits hepatitis B virus expression.J Hepatol. 2018 Mar;68(3):412-420. doi: 10.1016/j.jhep.2017.10.014. Epub 2017 Oct 25. J Hepatol. 2018. PMID: 29079285
-
Host Poly(A) Polymerases PAPD5 and PAPD7 Provide Two Layers of Protection That Ensure the Integrity and Stability of Hepatitis B Virus RNA.J Virol. 2021 Aug 25;95(18):e0057421. doi: 10.1128/JVI.00574-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34191584 Free PMC article.
-
Discovery and preclinical evaluations of GST-HG131, a novel HBV antigen inhibitor for the treatment of chronic hepatitis B infection.Bioorg Med Chem Lett. 2022 Nov 1;75:128977. doi: 10.1016/j.bmcl.2022.128977. Epub 2022 Sep 8. Bioorg Med Chem Lett. 2022. PMID: 36089112
-
Hepatitis B Gene Therapy Coming to Age.AIDS Rev. 2018 Apr-Jun;20(2):125-127. AIDS Rev. 2018. PMID: 29938706 Review.
-
Host RNA quality control as a hepatitis B antiviral target.Antiviral Res. 2021 Feb;186:104972. doi: 10.1016/j.antiviral.2020.104972. Epub 2020 Nov 24. Antiviral Res. 2021. PMID: 33242518 Review.
References
-
- World Health Organization. Hepatitis B Fact Sheet 2023. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (accessed on 4 December 2023).
LinkOut - more resources
Full Text Sources

