Genomic insights from Lactiplantibacillus plantarum BRD3A isolated from Atingba, a traditional fermented rice-based beverage and analysis of its potential for probiotic and antimicrobial activity against Methicillin-resistant Staphylococcus aureus
- PMID: 38628861
- PMCID: PMC11019378
- DOI: 10.3389/fmicb.2024.1357818
Genomic insights from Lactiplantibacillus plantarum BRD3A isolated from Atingba, a traditional fermented rice-based beverage and analysis of its potential for probiotic and antimicrobial activity against Methicillin-resistant Staphylococcus aureus
Abstract
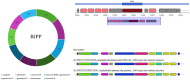
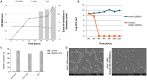
Lactiplantibacillus plantarum BRD3A was isolated from Atingba, a traditional fermented rice-based beverage of Manipur. Its genomic sequence has 13 contigs and its genome size is 3,320,817 bp with a guanine-cytosine (GC) ratio of 44.6%. It comprises 3185 genes including 3112 coding sequences (CDSs), 73 RNAs (including 66 tRNAs and others), and one clustered regularly interspaced short palindromic repeat (CRISPR) array. A comparative and phylogenetic analysis with the Lp. plantarum genome shows that this strain has close similarity with other Lp. plantarum strains and about 99% average nucleotide identity. Functional annotation using evolutionary genealogy of genes-non-supervised orthologous groups (EggNOG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) reveals genes associated with various biological processes such as metabolism, genetic information processing, and transport functions. Furthermore, the strain harbors bacteriocins like plantaricin E, Plantaricin F, and Enterocin X categorized under class IIb by the BAGEL4 database, indicating its potential antimicrobial properties. Additionally, AntiSMASH web server predicted four secondary regions-T3PKS, terpene, cyclic lactone inducer, and ribosomally synthesized and post-translationally modified peptide (RiPP)-suggesting an even higher antimicrobial potential. We validated the antimicrobial activity of Lp. plantarum BRD3A through in vitro experiments in which it exhibited promising bactericidal effects on methicillin-resistant Staphylococcus aureus, inhibiting their biofilm growth. These findings indicate the potential of Lp. plantarum BRD3A to be used as an alternative to conventional antibiotics.
Keywords: Lactobacillus; MRSA; antimicrobial; fermented rice beverages; genome; probiotic; secondary metabolites.
Copyright © 2024 Huidrom, Ngashangva, Khumlianlal, Sharma, Mukherjee and Devi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Alcock B. P., Huynh W., Chalil R., Smith K. W., Raphenya A. R., Wlodarski M. A., et al. . (2023). CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucl. Acids Res. 51, D690–D699. 10.1093/nar/gkac920 - DOI - PMC - PubMed
-
- Andrews S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed July 14, 2021).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

