Retinoic acid drives hair follicle stem cell activation via Wnt/β-catenin signalling in androgenetic alopecia
- PMID: 38629345
- PMCID: PMC11664453
- DOI: 10.1111/jdv.20000
Retinoic acid drives hair follicle stem cell activation via Wnt/β-catenin signalling in androgenetic alopecia
Abstract
Background: Depletion or permanent quiescence of the hair follicle stem cell (HFSC) pool underlies pathogenesis in androgenetic alopecia (AGA). Reactivation of quiescent HFSCs is considered an efficient treatment strategy for hair loss. The retinoic acid (RA) is critical to ensure stem cell homeostasis and function. However, little is known about whether RA regulates HFSC homeostasis. We aimed to investigate the impact of RA on HFSC homeostasis and the underlying mechanisms, in order to provide new potential targets for medical therapies of AGA.
Methods: Microdissected hair follicles from the occipital and frontal scalp in AGA were obtained for RNA sequencing analysis and test. The C57BL/6 mice model in telogen was established to investigate the effect of exogenous RA. Miniaturized hair follicles from frontal scalp were incubated with or without RA in hair follicle organ culture to test the effects on hair shaft elongation, hair cycling and HFSC activities. A strategy to characterize the effect of RA on HFSC in primary culture was developed to identify novel mechanisms that control HFSC activation. A clinical study was performed to test the efficacy of RA treatment in AGA patients.
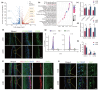
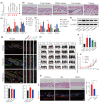
Results: RA signalling was inhibited in the course of AGA pathogenesis along with HFSC dysfunction. Hair regeneration was retarded in AGA miniaturized hair follicles with RA deficiency, but they tended to recover after treatment with RA. In addition, RA treatment during the telogen phase facilitated HFSC anagen entry and accelerated hair growth. Mechanistically, RA promoted hair growth by stimulating stem cells via Wnt/β-catenin signalling and accelerating the transition from a dormant to an activated state. Furthermore, a clinical study suggested that RA has obvious advantages in the early intervention of AGA by reactivating HFSCs.
Conclusions: Our study provides insights into the reactivation of HFSCs in AGA and provides potential targets for medical therapies.
© 2024 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




Similar articles
-
Therapeutic potential of isoproterenol in androgenetic alopecia: activation of hair follicle stem cells via the PI3K/AKT/β-Catenin signaling pathway.Stem Cell Res Ther. 2025 Jun 15;16(1):306. doi: 10.1186/s13287-025-04418-y. Stem Cell Res Ther. 2025. PMID: 40518527 Free PMC article.
-
Dahuang-Gancao decoction ameliorates testosterone-induced androgenetic alopecia in mice.J Ethnopharmacol. 2025 Feb 11;341:119347. doi: 10.1016/j.jep.2025.119347. Epub 2025 Jan 10. J Ethnopharmacol. 2025. PMID: 39800247
-
Exosome-derived long non-coding RNA AC010789.1 modified by FTO and hnRNPA2B1 accelerates growth of hair follicle stem cells against androgen alopecia by activating S100A8/Wnt/β-catenin signalling.Clin Transl Med. 2025 Jan;15(1):e70152. doi: 10.1002/ctm2.70152. Clin Transl Med. 2025. PMID: 39748192 Free PMC article.
-
Pathophysiological mechanisms of hair follicle regeneration and potential therapeutic strategies.Stem Cell Res Ther. 2025 Jun 15;16(1):302. doi: 10.1186/s13287-025-04420-4. Stem Cell Res Ther. 2025. PMID: 40518544 Free PMC article. Review.
-
Innovative strategies for the discovery of new drugs against androgenetic alopecia.Expert Opin Drug Discov. 2025 Apr;20(4):517-536. doi: 10.1080/17460441.2025.2473905. Epub 2025 Mar 11. Expert Opin Drug Discov. 2025. PMID: 40029254 Review.
Cited by
-
TWEAK regulates the functions of hair follicle stem cells via the Fn14-Wnt/β-catenin-CXCR4 signalling axis.Wound Repair Regen. 2025 May-Jun;33(3):e70032. doi: 10.1111/wrr.70032. Wound Repair Regen. 2025. PMID: 40325995 Free PMC article.
-
Tremella polysaccharide microneedles loaded with magnetic dental pulp stem cell intracellular vesicles used for androgenic alopecia.Stem Cell Res Ther. 2025 Mar 31;16(1):161. doi: 10.1186/s13287-025-04219-3. Stem Cell Res Ther. 2025. PMID: 40165226 Free PMC article.
-
UTX (KDM6A) promotes differentiation noncatalytically in somatic self-renewing epithelia.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2422971122. doi: 10.1073/pnas.2422971122. Epub 2025 May 15. Proc Natl Acad Sci U S A. 2025. PMID: 40372430
-
Microneedle-Based Approaches for Skin Disease Treatment.Nanomicro Lett. 2025 Feb 6;17(1):132. doi: 10.1007/s40820-025-01662-y. Nanomicro Lett. 2025. PMID: 39909997 Free PMC article. Review.
-
Molecular Signaling Pathways in Wound-Induced Hair-Follicle Neogenesis.Cells. 2025 Mar 16;14(6):440. doi: 10.3390/cells14060440. Cells. 2025. PMID: 40136689 Free PMC article. Review.
References
-
- Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. - PubMed
-
- Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57:9–17. - PubMed
-
- Vasserot AP, Geyfman M, Poloso NJ. Androgenetic alopecia: combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin Ther Targets. 2019;23:755–771. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

