Transmission of blaNDM in Enterobacteriaceae among animals, food and human
- PMID: 38629492
- PMCID: PMC11034458
- DOI: 10.1080/22221751.2024.2337678
Transmission of blaNDM in Enterobacteriaceae among animals, food and human
Abstract
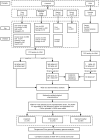
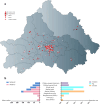
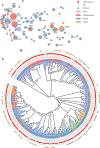
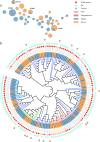
Despite carbapenems not being used in animals, carbapenem-resistant Enterobacterales (CRE), particularly New Delhi metallo-β-lactamase-producing CRE (NDM-CRE), are prevalent in livestock. Concurrently, the incidence of human infections caused by NDM-CRE is rising, particularly in children. Although a positive association between livestock production and human NDM-CRE infections at the national level was identified, the evidence of direct transmission of NDM originating from livestock to humans remains largely unknown. Here, we conducted a cross-sectional study in Chengdu, Sichuan Province, to examine the prevalence of NDM-CRE in chickens and pigs along the breeding-slaughtering-retail chains, in pork in cafeterias of schools, and in colonizations and infections from children's hospital and examined the correlation of NDM-CRE among animals, foods and humans. Overall, the blaNDM increases gradually along the chicken and pig breeding (4.70%/2.0%) -slaughtering (7.60%/22.40%) -retail (65.56%/34.26%) chains. The slaughterhouse has become a hotspot for cross-contamination and amplifier of blaNDM. Notably, 63.11% of pork from the school cafeteria was positive for blaNDM. The prevalence of blaNDM in intestinal and infection samples from children's hospitals was 21.68% and 19.80%, respectively. whole genome sequencing (WGS) analysis revealed the sporadic, not large-scale, clonal spread of NDM-CRE along the chicken and pig breeding-slaughtering-retail chain, with further spreading via IncX3-blaNDM plasmid within each stage of whole chains. Clonal transmission of NDM-CRE is predominant in children's hospitals. The IncX3-blaNDM plasmid was highly prevalent among animals and humans and accounted for 57.7% of Escherichia coli and 91.3% of Klebsiella pneumoniae. Attention should be directed towards the IncX3 plasmid to control the transmission of blaNDM between animals and humans.
Keywords: IncX3; NDM-CRE; child; food chain; livestock.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
