Verticillins: fungal epipolythiodioxopiperazine alkaloids with chemotherapeutic potential
- PMID: 38629495
- PMCID: PMC11409914
- DOI: 10.1039/d3np00068k
Verticillins: fungal epipolythiodioxopiperazine alkaloids with chemotherapeutic potential
Abstract
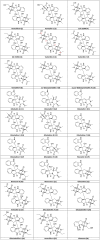
Covering: 1970 through June of 2023Verticillins are epipolythiodioxopiperazine (ETP) alkaloids, many of which possess potent, nanomolar-level cytotoxicity against a variety of cancer cell lines. Over the last decade, their in vivo activity and mode of action have been explored in detail. Notably, recent studies have indicated that these compounds may be selective inhibitors of histone methyltransferases (HMTases) that alter the epigenome and modify targets that play a crucial role in apoptosis, altering immune cell recognition, and generating reactive oxygen species. Verticillin A (1) was the first of 27 analogues reported from fungal cultures since 1970. Subsequent genome sequencing identified the biosynthetic gene cluster responsible for producing verticillins, allowing a putative pathway to be proposed. Further, molecular sequencing played a pivotal role in clarifying the taxonomic characterization of verticillin-producing fungi, suggesting that most producing strains belong to the genus Clonostachys (i.e., Bionectria), Bionectriaceae. Recent studies have explored the total synthesis of these molecules and the generation of analogues via both semisynthetic and precursor-directed biosynthetic approaches. In addition, nanoparticles have been used to deliver these molecules, which, like many natural products, possess challenging solubility profiles. This review summarizes over 50 years of chemical and biological research on this class of fungal metabolites and offers insights and suggestions on future opportunities to push these compounds into pre-clinical and clinical development.
Conflict of interest statement
The authors declare the following competing financial interest(s): NHO, HAR, and CJP are members of the Scientific Advisory Board of Clue Genetics, Inc. NHO is also a member of the Scientific Advisory Boards of Mycosynthetix, Inc. and Ionic Pharmaceuticals, LLC.
Figures








References
-
- Niego A. G. T. Lambert C. Mortimer P. Thongklang N. Rapior S. Grosse M. Schrey H. Charria-Girón E. Walker A. Hyde K. D. Stadler M. Fungal Diversity. 2023;121:95–137. doi: 10.1007/s13225-023-00520-9. - DOI
-
- Lax E., The mold in Dr. Florey's coat: the story of the penicillin miracle, Holt Paperbacks, New York, 2004
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

