Racial and ethnic differences in plasma biomarker eligibility for a preclinical Alzheimer's disease trial
- PMID: 38629508
- PMCID: PMC11180863
- DOI: 10.1002/alz.13803
Racial and ethnic differences in plasma biomarker eligibility for a preclinical Alzheimer's disease trial
Abstract
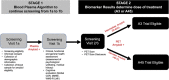
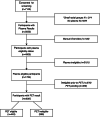
Introduction: In trials of amyloid-lowering drugs for Alzheimer's disease (AD), differential eligibility may contribute to under-inclusion of racial and ethnic underrepresented groups. We examined plasma amyloid beta 42/40 and positron emission tomography (PET) amyloid eligibility for the ongoing AHEAD Study preclinical AD program (NCT04468659).
Methods: Univariate logistic regression models were used to examine group differences in plasma and PET amyloid screening eligibility.
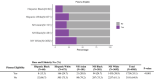
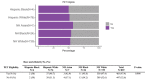
Results: Of 4905 participants screened at time of analysis, 1724 were plasma eligible to continue in screening: 13.3% Hispanic Black, 24.7% Hispanic White, 20.8% non-Hispanic (NH) Asian, 24.7% NH Black, and 38.9% NH White. Plasma eligibility differed across groups in models controlling for covariates (odds ratio from 1.9 to 4.0 compared to the NH White reference group, P < 0.001). Among plasma eligible participants, PET eligibility did not differ by group.
Discussion: These results suggest that prevalence of brain amyloid pathology differed, but that eligibility based on plasma was equally effective across racial and ethnic group members.
Highlights: Plasma amyloid eligibility is lower in underrepresented racial and ethnic groups. In plasma eligible adults, positron emission tomography eligibility rates are similar across race and ethnicity. Plasma biomarker tests may be similarly effective across racial and ethnic groups.
Keywords: amyloid; biomarker; ethnicity; plasma; positron emission tomography; race.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
DPMH has received funding from the American Heart Association. AL, OL, and LS are employees of the Keck School of Medicine of USC. JBB, KEY, VV, TW, and PBV and are paid employees of C2N Diagnostics, and collectively have research grants from the NIH, BrightFocus Foundation, GHR Foundation, and Alzheimer's Drug Discovery Foundation. SD and MI are paid employees of Eisai. RAR and RR have grants from the NIH. RR has received grants from Eisai, Eli Lilly, the Alzheimer's Association, and the American Heart Association. KAJ has grants from the NIH and GHR Foundation, and has consulted for Novartis, Janssen, and Merck. CG has nothing to disclose. GJ‐M has received support from C2N Diagnostics, Eisai Co, Ltd, and the NIH. PSA has research grants from Eisai, NIH, Lilly, the Alzheimer's Association, and Janssen and serves as a consultant for Merck, Bristol Myers Squibb, Switch Therapeutics, Roche, Arrowhead, ImmunoBrain, Checkpoint, Biogen, Abbvie, Genetech, and NewAmsterdam Pharma. JDG has received consultancy fees from SiteRx, outside the submitted work. RAS has grants from the NIH, the Alzheimer's Association, the GHR Foundation, Eli Lilly, and Eisai, and has consulted for Abbvie, AC Immune, Acumen, Alector, Alnylam, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Shionogi, Vigil Neuroscience, Ionis, Vaxxinity, and Bristol Myers Squibb. Author disclosures are available in the supporting information.
Figures