Evolution of H7N9 highly pathogenic avian influenza virus in the context of vaccination
- PMID: 38629574
- PMCID: PMC11060016
- DOI: 10.1080/22221751.2024.2343912
Evolution of H7N9 highly pathogenic avian influenza virus in the context of vaccination
Abstract
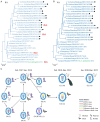
Human infections with the H7N9 influenza virus have been eliminated in China through vaccination of poultry; however, the H7N9 virus has not yet been eradicated from poultry. Carefully analysis of H7N9 viruses in poultry that have sub-optimal immunity may provide a unique opportunity to witness the evolution of highly pathogenic avian influenza virus in the context of vaccination. Between January 2020 and June 2023, we isolated 16 H7N9 viruses from samples we collected during surveillance and samples that were sent to us for disease diagnosis. Genetic analysis indicated that these viruses belonged to a single genotype previously detected in poultry. Antigenic analysis indicated that 12 of the 16 viruses were antigenically close to the H7-Re4 vaccine virus that has been used since January 2022, and the other four viruses showed reduced reactivity with the vaccine. Animal studies indicated that all 16 viruses were nonlethal in mice, and four of six viruses showed reduced virulence in chickens upon intranasally inoculation. Importantly, the H7N9 viruses detected in this study exclusively bound to the avian-type receptors, having lost the capacity to bind to human-type receptors. Our study shows that vaccination slows the evolution of H7N9 virus by preventing its reassortment with other viruses and eliminates a harmful characteristic of H7N9 virus, namely its ability to bind to human-type receptors.
Keywords: Avian influenza virus; H7N9; evolution; receptor-binding properties; pathogenicity; antigenicity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Liu L, Yang H, Guo F, et al. . Emergence of H5N1 highly pathogenic avian influenza in Democratic People's Republic of Korea. J Integr Agric. 2022;21(5):1534–1538. doi:10.1016/S2095-3119(21)63829-7 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
