FoxG1 as a Potential Therapeutic Target for Alzheimer's Disease: Modulating NLRP3 Inflammasome via AMPK/mTOR Autophagy Pathway
- PMID: 38630150
- PMCID: PMC11023968
- DOI: 10.1007/s10571-024-01467-4
FoxG1 as a Potential Therapeutic Target for Alzheimer's Disease: Modulating NLRP3 Inflammasome via AMPK/mTOR Autophagy Pathway
Abstract
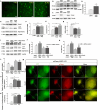
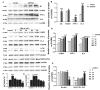
An increasing body of research suggests that promoting microglial autophagy hinders the neuroinflammation initiated though the NLRP3 inflammasome activation in Alzheimer's disease (AD). The function of FoxG1, a crucial transcription factor involved in cell survival by regulating mitochondrial function, remains unknown during the AD process and neuroinflammation occurs. In the present study, we firstly found that Aβ peptides induced AD-like neuroinflammation upregulation and downregulated the level of autophagy. Following low-dose Aβ25-35 stimulation, FoxG1 expression and autophagy exhibited a gradual increase. Nevertheless, with high-concentration Aβ25-35 treatment, progressive decrease in FoxG1 expression and autophagy levels as the concentration of Aβ25-35 escalated. In addition, FoxG1 has a positive effect on cell viability and autophagy in the nervous system. In parallel with the Aβ25-35 stimulation, we employed siRNA to decrease the expression of FoxG1 in N2A cells. A substantial reduction in autophagy level (Beclin1, LC3II, SQSTM1/P62) and a notable growth in inflammatory response (NLRP3, TNF-α, and IL-6) were observed. In addition, we found FoxG1 overexpression owned the effect on the activation of AMPK/mTOR autophagy pathway and siRNA-FoxG1 successfully abolished this effect. Lastly, FoxG1 suppressed the NLRP3 inflammasome and enhanced the cognitive function in AD-like mouse model induced by Aβ25-35. Confirmed by cellular and animal experiments, FoxG1 suppressed NLRP3-mediated neuroinflammation, which was strongly linked to autophagy regulated by AMPK/mTOR. Taken together, FoxG1 may be a critical node in the pathologic progression of AD and has the potential to serve as therapeutic target.
Keywords: AMPK/mTOR; Alzheimer’s disease; Autophagy; FoxG1; NLRP3; β-amyloid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction.Biomed Pharmacother. 2020 Oct;130:110575. doi: 10.1016/j.biopha.2020.110575. Epub 2020 Aug 5. Biomed Pharmacother. 2020. PMID: 32768883
-
Lychee seed polyphenol protects the blood-brain barrier through inhibiting Aβ(25-35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice.Phytother Res. 2021 Feb;35(2):954-973. doi: 10.1002/ptr.6849. Epub 2020 Sep 6. Phytother Res. 2021. PMID: 32893437
-
Targeting CB2R in astrocytes for Parkinson's disease therapy: unraveling the Foxg1-mediated neuroprotective mechanism through autophagy-mediated NLRP3 degradation.J Neuroinflammation. 2023 Dec 19;20(1):304. doi: 10.1186/s12974-023-02989-2. J Neuroinflammation. 2023. PMID: 38110963 Free PMC article.
-
Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer's Disease.Neurochem Res. 2020 Nov;45(11):2560-2572. doi: 10.1007/s11064-020-03121-z. Epub 2020 Sep 14. Neurochem Res. 2020. PMID: 32929691 Review.
-
NLRP3 inflammasome in Alzheimer's disease: molecular mechanisms and emerging therapies.Front Immunol. 2025 Apr 7;16:1583886. doi: 10.3389/fimmu.2025.1583886. eCollection 2025. Front Immunol. 2025. PMID: 40260242 Free PMC article. Review.
Cited by
-
Synergistic Autophagy-Related Mechanisms of Protection Against Brain Aging and AD: Cellular Pathways and Therapeutic Strategies.Pharmaceuticals (Basel). 2025 Jun 1;18(6):829. doi: 10.3390/ph18060829. Pharmaceuticals (Basel). 2025. PMID: 40573225 Free PMC article. Review.
-
Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention.Molecules. 2024 Oct 12;29(20):4841. doi: 10.3390/molecules29204841. Molecules. 2024. PMID: 39459209 Free PMC article. Review.
-
Decoding microglial immunometabolism: a new frontier in Alzheimer's disease research.Mol Neurodegener. 2025 Mar 27;20(1):37. doi: 10.1186/s13024-025-00825-0. Mol Neurodegener. 2025. PMID: 40149001 Free PMC article. Review.
-
FOXG1 promotes osteogenesis of bone marrow-derived mesenchymal stem cells by activating autophagy through regulating USP14.Commun Biol. 2025 Jan 15;8(1):59. doi: 10.1038/s42003-024-07429-2. Commun Biol. 2025. PMID: 39814826 Free PMC article.
-
Microglial NLRP3 Inflammasomes in Alzheimer's Disease Pathogenesis: From Interaction with Autophagy/Mitophagy to Therapeutics.Mol Neurobiol. 2025 Jun;62(6):7124-7143. doi: 10.1007/s12035-025-04758-z. Epub 2025 Feb 14. Mol Neurobiol. 2025. PMID: 39951189 Review.
References
-
- Abad-Jiménez Z, López-Domènech S, García-Gargallo C, Vezza T, Gómez-Abril SÁ, Morillas C, Díaz-Pozo P, Falcón R, Bañuls C, Víctor VM, Rocha M (2022) Roux-en-Y gastric bypass modulates AMPK, autophagy and inflammatory response in leukocytes of obese patients. Biomedicines. 10.3390/biomedicines10020430 - PMC - PubMed
-
- Adesina AM, Veo BL, Courteau G, Mehta V, Wu X, Pang K, Liu Z, Li X-N, Peters L (2015) FOXG1 expression shows correlation with neuronal differentiation in cerebellar development, aggressive phenotype in medulloblastomas, and survival in a xenograft model of medulloblastoma. Hum Pathol 46(12):1859–1871. 10.1016/j.humpath.2015.08.003 - PubMed
-
- Aizawa S, Hoki M, Yamamuro Y (2017) Lactoferrin promotes autophagy via AMP-activated protein kinase activation through low-density lipoprotein receptor-related protein 1. Biochem Biophys Res Commun 493(1):509–513. 10.1016/j.bbrc.2017.08.160 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

