Population Pharmacokinetics of Adalimumab in Juvenile Idiopathic Arthritis Patients: A Retrospective Cohort Study Using Clinical Care Data
- PMID: 38630199
- PMCID: PMC11192828
- DOI: 10.1007/s40272-024-00629-7
Population Pharmacokinetics of Adalimumab in Juvenile Idiopathic Arthritis Patients: A Retrospective Cohort Study Using Clinical Care Data
Abstract
Background and objective: Juvenile idiopathic arthritis (JIA) is a chronic autoimmune disorder that primarily affects the joints in children. Notably, it is known to co-occur with uveitis. Adalimumab, a monoclonal anti-TNF antibody, is effective in treating both conditions. A deeper understanding of the pharmacokinetics (PK) of adalimumab in JIA is crucial to advance in more personalized treatment approaches. The objective of this study is to evaluate the population PK profile of adalimumab in JIA and to explain causes for its variability.
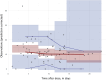
Materials and methods: Adalimumab and antidrug antibody concentrations were retrospectively retrieved from the charts of patients with JIA. Initially, five literature-based population PK models of adalimumab were evaluated to assess their ability to describe the observed concentration-time profiles in the JIA cohort. These models included one specifically for the pediatric Crohn's disease population and four derived from studies in adult populations in healthy subjects and rheumatoid arthritis patients. Subsequently, a novel population PK model tailored to the JIA population was developed using NONMEM software. Monte Carlo simulations were then conducted utilizing the final PK model to visualize the concentration-time profile of adalimumab in patients with JIA and the impact of covariates.
Results: A cohort of 50 patients with JIA with 78 available adalimumab samples was assessed. The mean age was 11.8 ± 3.9 years, with a median body weight of 49 kg (interquartile range 29.4-59.8 kg). All literature models adequately described the concentration-time profiles in JIA. The best model, which was developed in patients with rheumatoid arthritis during the maintenance phase of treatment, served as a basis for estimating clearance in JIA, resulting in a value of 0.37 L per day per 70 kg. Patient body weight, antidrug antibodies, methotrexate use, CRP level, and comorbidity of uveitis were found to have a significant impact on adalimumab clearance, and these reduced the inter-patient variability from 58.6 to 28.0%. On steady state in the simulated patient population, the mean trough level was 7.4 ± 5.5 mg/L. The two dosing regimens of 20 and 40 mg every other week, based on patients' body weight, resulted in comparable simulated overall drug exposure.
Conclusions: Five literature models effectively described adalimumab PK in this pediatric cohort, highlighting the potential for extrapolating existing models to the pediatric population. The new JIA model confirmed the effect of several known covariates and found a novel association for drug clearance with methotrexate use (lower) and uveitis (higher), which might have clinical relevance for personalized dosing in JIA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

