Drug Survival of IL-17 and IL-23 Inhibitors for Psoriasis: A Systematic Review and Meta-Analysis
- PMID: 38630365
- PMCID: PMC11190018
- DOI: 10.1007/s40265-024-02028-1
Drug Survival of IL-17 and IL-23 Inhibitors for Psoriasis: A Systematic Review and Meta-Analysis
Abstract
Background and objective: The most recently approved biologics for moderate-to-severe psoriasis are the interleukin (IL)-17 and IL-23 inhibitors. Drug survival is a frequently used outcome to assess drug performance in practice. An overview of the available drug survival studies regarding IL-17 and IL-23 inhibitors is lacking. Therefore, our objective was to assess the drug survival of IL-17 and IL-23 inhibitors for psoriasis.
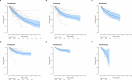
Methods: A search of PubMed, Embase, Cochrane Library and Web of Science was conducted (last search 27 December, 2023). Inclusion criteria were (1) cohort study; (2) patients aged ≥ 18 years with plaque psoriasis; and (3) evaluation of drug survival of at least one of the IL-17 and IL-23 inhibitors. Exclusion criteria were: primary focus on patients with psoriatic arthritis, fewer than ten study subjects and another language than English. The Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline was followed. Survival probabilities at monthly intervals were extracted from Kaplan-Meier curves using a semi-automated tool. Data were pooled using a non-parametric random-effects model to retrieve distribution-free summary survival curves. Summary drug survival curves were constructed per biologic for different discontinuation reasons: overall, ineffectiveness and adverse events, and split for the effect modifier biologic naivety. Results were analysed separately for registry/electronic health record data and for pharmacy/claims data.
Results: A total of 69 studies aggregating drug survival outcomes of 48,704 patients on secukinumab, ixekizumab, brodalumab, guselkumab, risankizumab, and tildrakizumab were included. Summary drug survival estimates of registry/electronic health record studies for overall, ineffectiveness and adverse event related drug survival were high (all point estimates ≥ 0.8 at year 1) for included biologics, with highest estimates for guselkumab and risankizumab. All estimates for drug survival were higher in biologic naive than in experienced patients. Estimates of pharmacy/claims databases were substantially lower than estimates from the primary analyses based on registry/electronic health record data.
Conclusions: This meta-analysis showed that the investigated IL-17 and IL-23 inhibitors had high drug survival rates, with highest rates for guselkumab and risankizumab drug survival. We showed that effect modifiers such as biologic naivety, and the source of data used (registry/electronic health record data vs pharmacy/claims databases) is relevant when interpreting drug survival studies.
© 2024. The Author(s).
Conflict of interest statement
Sarah E. Thomas carries out clinical trials for Janssen and Novartis and received speaking fees from Eli Lilly and AbbVie. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology, Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands. Liana Barenbrug and Gerjon Hannink have no conflicts of interest that are directly relevant to the content of this article. Marieke M.B. Seyger received grants from/was involved in clinical trials from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma and Pfizer. She served as a consultant for AbbVie, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer and UCB; fees were paid directly to the Department of Dermatology, Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands. Elke M.G.J. de Jong has received research grants for the independent research fund of the Department of Dermatology, Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands, from AbbVie, BMS, Janssen Pharmaceutica, Leo Pharma, Novartis and UCB for research on psoriasis and has acted as a consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis or eczema including AbbVie, Amgen, Almirall, Boehringer Ingelheim, Celgene, Galapagos, Janssen Pharmaceutica, Leo Pharma, Lilly, Novartis, Sanofi and UCB. All funding is not personal but goes directly to the Department of Dermatology, Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands. Juul M.P.A. van den Reek carried out clinical trials for AbbVie, Celgene, Almirall and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma, Novartis, UCB and Eli Lilly and reimbursement for attending or chairing a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the Department of Dermatology, Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands.
Figures


References
-
- EMA. Bimzelx (bimekizumab). 2021. https://www.ema.europa.eu/en/documents/overview/bimzelx-epar-medicine-ov.... Accessed 9 Apr 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

