Targeting inflammation in perivascular cells and neuroimmune interactions for treating kidney disease
- PMID: 38630367
- PMCID: PMC11116252
- DOI: 10.1007/s10157-024-02494-7
Targeting inflammation in perivascular cells and neuroimmune interactions for treating kidney disease
Abstract
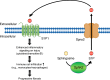
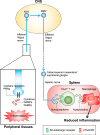
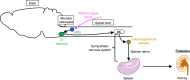
Inflammation plays a crucial role in the pathophysiology of various kidney diseases. Kidney perivascular cells (pericytes/fibroblasts) are responsible for producing proinflammatory molecules, promoting immune cell infiltration, and enhancing inflammation. Vascular adhesion protein-1, expressed in kidney perivascular cells, is an ectoenzyme that catalyzes the oxidative deamination of primary amines with the production of hydrogen peroxide in the extracellular space. Our study demonstrated that blocking this enzyme suppressed hydrogen peroxide production and neutrophil infiltration, thereby reducing renal ischemia-reperfusion injury. Sphingosine 1-phosphate (S1P) signaling was also observed to play an essential role in the regulation of perivascular inflammation. S1P, which is produced in kidney perivascular cells, is transported into the extracellular space via spinster homolog 2, and then binds to S1P receptor-1 expressed in perivascular cells. Upon injury, inflammatory signaling in perivascular cells is enhanced by this pathway, thereby promoting immune cell infiltration and subsequent fibrosis. Furthermore, inhibition of S1P transport by spinster homolog 2 reduces kidney fibrosis. Hypoxia-inducible factor-prolyl hydroxylase inhibitors can restore the capacity for erythropoietin production in kidney perivascular cells. Animal data suggested that these drugs could also alleviate kidney and lipid inflammation although the precise mechanism is still unknown. Neuroimmune interactions have been attracting significant attention due to their potential to benefit patients with inflammatory diseases. Vagus nerve stimulation is one of the most promising strategies for harnessing neuroimmune interactions and attenuating inflammation associated with various diseases, including kidney disease. Using cutting-edge tools, the vagal afferents-C1 neurons-sympathetic nervous system-splenic nerve-spleen-kidney axis responsible for kidney protection induced by vagus nerve stimulation was identified in our study. Further research is required to decipher other crucial systems that control kidney inflammation and to determine whether these novel strategies can be applied to patients with kidney disease.
Keywords: Acute kidney injury; Chronic kidney disease; Fibroblasts; Neuroimmune interactions; Pericytes; Vagus nerve stimulation.
© 2024. The Author(s).
Figures

References
-
- Levey AS, James MT. Acute kidney injury. Ann Int Med. 2017;167(9):ITC66-ITC80. 10.7326/AITC201711070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

