Spontaneous breathing trial with pressure support on positive end-expiratory pressure and extensive use of non-invasive ventilation versus T-piece in difficult-to-wean patients from mechanical ventilation: a randomized controlled trial
- PMID: 38630372
- PMCID: PMC11024068
- DOI: 10.1186/s13613-024-01290-6
Spontaneous breathing trial with pressure support on positive end-expiratory pressure and extensive use of non-invasive ventilation versus T-piece in difficult-to-wean patients from mechanical ventilation: a randomized controlled trial
Abstract
Background: The aim of this study is to assess whether a strategy combining spontaneous breathing trial (SBT) with both pressure support (PS) and positive end-expiratory pressure (PEEP) and extended use of post-extubation non-invasive ventilation (NIV) (extensively-assisted weaning) would shorten the time until successful extubation as compared with SBT with T-piece (TP) and post-extubation NIV performed in selected patients as advocated by guidelines (standard weaning), in difficult-to-wean patients from mechanical ventilation.
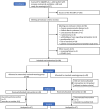
Methods: The study is a single-center prospective open label, randomized controlled superiority trial with two parallel groups and balanced randomization with a 1:1 ratio. Eligible patients were intubated patients mechanically ventilated for more than 24 h who failed their first SBT using TP. In the extensively-assisted weaning group, SBT was performed with PS (7 cmH2O) and PEEP (5 cmH2O). In case of SBT success, an additional SBT with TP was performed. Failure of this SBT-TP was an additional criterion for post-extubation NIV in this group in addition to other recommended criteria. In the standard weaning group, SBT was performed with TP, and NIV was performed according to international guidelines. The primary outcome criterion was the time between inclusion and successful extubation evaluated with a Cox model with adjustment on randomization strata.
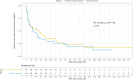
Results: From May 2019 to March 2023, 98 patients were included and randomized in the study (49 in each group). Four patients were excluded from the intention-to-treat population (2 in both groups); therefore, 47 patients were analyzed in each group. The extensively-assisted weaning group had a higher median age (68 [58-73] vs. 62 [55-71] yrs.) and similar sex ratio (62% male vs. 57%). Time until successful extubation was not significantly different between extensively-assisted and standard weaning groups (median, 172 [50-436] vs. 95 [47-232] hours, Cox hazard ratio for successful extubation, 0.88 [95% confidence interval: 0.55-1.42] using the standard weaning group as a reference; p = 0.60). All secondary outcomes were not significantly different between groups.
Conclusion: An extensively-assisted weaning strategy did not lead to a shorter time to successful extubation than a standard weaning strategy. Trial registration The trial was registered on ClinicalTrials.gov (NCT03861117), on March 1, 2019, before the inclusion of the first patient. https://clinicaltrials.gov/study/NCT03861117 .
Keywords: Difficult weaning; Positive end-expiratory pressure; Pressure support; Spontaneous breathing trial; T-piece.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, et al. Liberation from mechanical ventilation in critically Ill adults: an official american college of chest physicians/american thoracic society clinical practice guideline. Chest. 2017;151:166–180. doi: 10.1016/j.chest.2016.10.036. - DOI - PubMed
-
- Subirà C, Hernández G, Vázquez A, Rodríguez-García R, González-Castro A, García C, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321:2175. doi: 10.1001/jama.2019.7234. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

