Immune Checkpoint Inhibitor Use During Pregnancy and Outcomes in Pregnant Individuals and Newborns
- PMID: 38630478
- PMCID: PMC11024778
- DOI: 10.1001/jamanetworkopen.2024.5625
Immune Checkpoint Inhibitor Use During Pregnancy and Outcomes in Pregnant Individuals and Newborns
Abstract
Importance: With the widespread use of immune checkpoint inhibitors (ICIs), concerns about their pregnancy outcomes through maternal exposure have emerged, and clinical comparative data are lacking.
Objective: To assess the risk of pregnancy-, fetal-, and/or newborn-related adverse outcomes associated with exposure to ICIs compared with exposure to other anticancer agents.
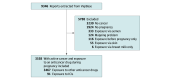
Design, setting, and participants: In this cohort study, all reports mentioning a pregnancy-related condition and an antineoplastic agent (Anatomical Therapeutic Chemical classification group L01) used for a cancer indication registered in the World Health Organization international pharmacovigilance database VigiBase up to June 26, 2022, were extracted.
Exposure: Anticancer agents, including ICIs, used during pregnancy for a cancer indication. Immune checkpoint inhibitors included blockers of programmed cell death 1 (PD1) or its ligand (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA4).
Main outcomes and measures: The main outcome was the reporting odds ratio (ROR) for maternal, fetal, or newborn complications in patients treated with ICIs vs any other anticancer drug. Adverse events, categorized into 45 individual maternofetal adverse outcomes, were directly mapped to Medical Dictionary for Regulatory Activities preferred terms in VigiBase.
Results: A total of 3558 reports (ICI: 91 [2.6%]; other anticancer drugs: 3467 [97.4%]) were included in the analysis. In the ICI group, most reports were from the US (60 [65.9%]), and the mean (SD) patient age was 28.9 (10.2) years; in 24 of 55 reports with data on cancer type (43.6%), patients were treated for melanoma. The molecules involved in the ICI group were anti-PD1 (58 reports [63.7%]), anti-PD1 plus anti-CTLA4 (15 [16.5%]), anti-CTLA4 (13 [14.3%]), anti-PD-L1 (4 [4.4%]), and anti-PD1 plus anti-lymphocyte activation gene 3 (1 [1.1%]). An ICI was used in combination with a non-ICI anticancer agent in 10 participants (11.0%). Compared with other anticancer drugs, none of the 45 adverse outcomes identified were overreported in the group exposed to ICIs. However, preterm birth was significantly overreported for the anti-PD1 plus anti-CTLA4 combination compared with other anticancer drugs (12 of 15 [80.0%] vs 793 of 3452 [23.0%]; ROR, 13.87; 95% CI, 3.90-49.28; P < .001) but not for anti-PD-L1 or anti-CTLA4 monotherapy. Three reports of possibly immune-related maternofetal events were identified: 1 case of maternal antiphospholipid syndrome leading to spontaneous abortion, 1 case of pneumonitis leading to neonatal respiratory distress syndrome and death, and 1 case of transient congenital hypothyroidism.
Conclusions and relevance: In this cohort study of 91 individuals exposed to ICIs during pregnancy, ICI exposure was not associated with overreporting of specific adverse pregnancy, fetal, and/or newborn outcomes compared with other anticancer treatments. However, due to possible rare immune-related neonatal adverse events, ICI use in pregnant women should be avoided when possible, especially the anti-PD1 plus anti-CTLA4 combination.
Conflict of interest statement
Figures


Comment in
-
Pregnancy and Cancer-Navigating Impossible Decisions.JAMA Netw Open. 2024 Apr 1;7(4):e246486. doi: 10.1001/jamanetworkopen.2024.6486. JAMA Netw Open. 2024. PMID: 38630480 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

