Extended Clopidogrel Monotherapy vs DAPT in Patients With Acute Coronary Syndromes at High Ischemic and Bleeding Risk: The OPT-BIRISK Randomized Clinical Trial
- PMID: 38630489
- PMCID: PMC11024736
- DOI: 10.1001/jamacardio.2024.0534
Extended Clopidogrel Monotherapy vs DAPT in Patients With Acute Coronary Syndromes at High Ischemic and Bleeding Risk: The OPT-BIRISK Randomized Clinical Trial
Abstract
Importance: Purinergic receptor P2Y12 (P2Y12) inhibitor monotherapy after a certain period of dual antiplatelet therapy (DAPT) may be an attractive option of maintenance antiplatelet treatment for patients undergoing percutaneous coronary intervention (PCI) who are at both high bleeding and ischemic risk (birisk).
Objective: To determine if extended P2Y12 inhibitor monotherapy with clopidogrel is superior to ongoing DAPT with aspirin and clopidogrel after 9 to 12 months of DAPT after PCI in birisk patients with acute coronary syndromes (ACS).
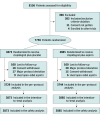
Design, setting, and participants: This was a multicenter, double-blind, placebo-controlled, randomized clinical trial including birisk patients with ACS who had completed 9 to 12 months of DAPT after drug-eluting stent implantation and were free from adverse events for at least 6 months at 101 China centers between February 2018 and December 2020. Study data were analyzed from April 2023 to May 2023.
Interventions: Patients were randomized either to clopidogrel plus placebo or clopidogrel plus aspirin for an additional 9 months.
Main outcomes and measures: The primary end point was Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding 9 months after randomization. The key secondary end point was major adverse cardiac and cerebral events (MACCE; the composite of all-cause death, myocardial infarction, stroke or clinically driven revascularization). The primary end point was tested for superiority, and the MACCE end point was tested for sequential noninferiority and superiority.
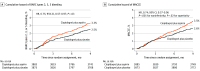
Results: A total of 7758 patients (mean [SD] age, 64.8 [9.0] years; 4575 male [59.0%]) were included in this study. The primary end point of BARC types 2, 3, or 5 bleeding occurred in 95 of 3873 patients (2.5%) assigned to clopidogrel plus placebo and 127 of 3885 patients (3.3%) assigned to clopidogrel plus aspirin (hazard ratio [HR], 0.75; 95% CI, 0.57-0.97; difference, -0.8%; 95% CI, -1.6% to -0.1%; P = .03). The incidence of MACCE was 2.6% (101 of 3873 patients) in the clopidogrel plus placebo group and 3.5% (136 of 3885 patients) in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96; difference, -0.9%; 95% CI, -1.7% to -0.1%; P < .001 for noninferiority; P = .02 for superiority).
Conclusions and relevance: Among birisk patients with ACS who completed 9 to 12 months of DAPT after drug-eluting stent implantation and were free from adverse events for at least 6 months before randomization, an extended 9-month clopidogrel monotherapy regimen was superior to continuing DAPT with clopidogrel in reducing clinically relevant bleeding without increasing ischemic events.
Trial registration: ClinicalTrials.gov Identifier: NCT03431142.
Conflict of interest statement
Figures
Comment on
-
Clopidogrel Monotherapy for Double-Risk Acute Coronary Syndrome.JAMA Cardiol. 2024 Jun 1;9(6):532-533. doi: 10.1001/jamacardio.2024.0547. JAMA Cardiol. 2024. PMID: 38630487 No abstract available.
References
-
- Lawton JS, Tamis-Holland JE, Bangalore S, et al. . 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145(3):e4-e17. doi:10.1161/CIR.0000000000001039 - DOI - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC-focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi:10.1093/eurheartj/ehx419 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous