Lipoarabinomannan modification as a source of phenotypic heterogeneity in host-adapted Mycobacterium abscessus isolates
- PMID: 38630725
- PMCID: PMC11046677
- DOI: 10.1073/pnas.2403206121
Lipoarabinomannan modification as a source of phenotypic heterogeneity in host-adapted Mycobacterium abscessus isolates
Abstract
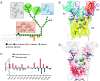
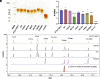
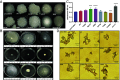
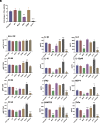
Mycobacterium abscessus is increasingly recognized as the causative agent of chronic pulmonary infections in humans. One of the genes found to be under strong evolutionary pressure during adaptation of M. abscessus to the human lung is embC which encodes an arabinosyltransferase required for the biosynthesis of the cell envelope lipoglycan, lipoarabinomannan (LAM). To assess the impact of patient-derived embC mutations on the physiology and virulence of M. abscessus, mutations were introduced in the isogenic background of M. abscessus ATCC 19977 and the resulting strains probed for phenotypic changes in a variety of in vitro and host cell-based assays relevant to infection. We show that patient-derived mutational variations in EmbC result in an unexpectedly large number of changes in the physiology of M. abscessus, and its interactions with innate immune cells. Not only did the mutants produce previously unknown forms of LAM with a truncated arabinan domain and 3-linked oligomannoside chains, they also displayed significantly altered cording, sliding motility, and biofilm-forming capacities. The mutants further differed from wild-type M. abscessus in their ability to replicate and induce inflammatory responses in human monocyte-derived macrophages and epithelial cells. The fact that different embC mutations were associated with distinct physiologic and pathogenic outcomes indicates that structural alterations in LAM caused by nonsynonymous nucleotide polymorphisms in embC may be a rapid, one-step, way for M. abscessus to generate broad-spectrum diversity beneficial to survival within the heterogeneous and constantly evolving environment of the infected human airway.
Keywords: Mycobacterium abscessus; biofilm; immunomodulation; lipoarabinomannan; nontuberculous mycobacteria.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Martiniano S. L., Nick J. A., Daley C. L., Nontuberculous mycobacterial infections in cystic fibrosis. Clin. Chest Med. 43, 697–716 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

