RIPK3 deficiency blocks R-2-hydroxyglutarate-induced necroptosis in IDH-mutated AML cells
- PMID: 38630819
- PMCID: PMC11023509
- DOI: 10.1126/sciadv.adi1782
RIPK3 deficiency blocks R-2-hydroxyglutarate-induced necroptosis in IDH-mutated AML cells
Abstract
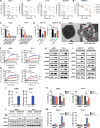
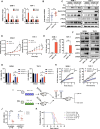
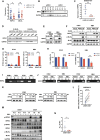
Mutant isocitrate dehydrogenases (IDHs) produce R-2-hydroxyglutarate (R-2HG), which inhibits the growth of most acute myeloid leukemia (AML) cells. Here, we showed that necroptosis, a form of programmed cell death, contributed to the antileukemia activity of R-2HG. Mechanistically, R-2HG competitively inhibited the activity of lysine demethylase 2B (KDM2B), an α-ketoglutarate-dependent dioxygenase. KDM2B inhibition increased histone 3 lysine 4 trimethylation levels and promoted the expression of receptor-interacting protein kinase 1 (RIPK1), which consequently caused necroptosis in AML cells. The expression of RIPK3 was silenced because of DNA methylation in IDH-mutant (mIDH) AML cells, resulting in R-2HG resistance. Decitabine up-regulated RIPK3 expression and repaired endogenous R-2HG-induced necroptosis pathway in mIDH AML cells. Together, R-2HG induced RIPK1-dependent necroptosis via KDM2B inhibition in AML cells. The loss of RIPK3 protected mIDH AML cells from necroptosis. Restoring RIPK3 expression to exert R-2HG's intrinsic antileukemia effect will be a potential therapeutic strategy in patients with AML.
Figures

References
-
- Loenarz C., Schofield C. J., Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem. Sci. 36, 7–18 (2011). - PubMed
-
- Molenaar R. J., Thota S., Nagata Y., Patel B., Clemente M., Przychodzen B., Hirsh C., Viny A. D., Hosano N., Bleeker F. E., Meggendorfer M., Alpermann T., Shiraishi Y., Chiba K., Tanaka H., van Noorden C. J. F., Radivoyevitch T., Carraway H. E., Makishima H., Miyano S., Sekeres M. A., Ogawa S., Haferlach T., Maciejewski J. P., Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms. Leukemia 29, 2134–2142 (2015). - PMC - PubMed
-
- Cancer Genome Atlas Research Network, Ley T. J., Miller C., Ding L., Raphael B. J., Mungall A. J., Robertson A., Hoadley K., Triche T. J. Jr., Laird P. W., Baty J. D., Fulton L. L., Fulton R., Heath S. E., Kalicki-Veizer J., Kandoth C., Klco J. M., Koboldt D. C., Kanchi K. L., Kulkarni S., Lamprecht T. L., Larson D. E., Lin L., Lu C., McLellan M. D., McMichael J. F., Payton J., Schmidt H., Spencer D. H., Tomasson M. H., Wallis J. W., Wartman L. D., Watson M. A., Welch J., Wendl M. C., Ally A., Balasundaram M., Birol I., Butterfield Y., Chiu R., Chu A., Chuah E., Chun H. J., Corbett R., Dhalla N., Guin R., He A., Hirst C., Hirst M., Holt R. A., Jones S., Karsan A., Lee D., Li H. I., Marra M. A., Mayo M., Moore R. A., Mungall K., Parker J., Pleasance E., Plettner P., Schein J., Stoll D., Swanson L., Tam A., Thiessen N., Varhol R., Wye N., Zhao Y., Gabriel S., Getz G., Sougnez C., Zou L., Leiserson M. D., Vandin F., Wu H. T., Applebaum F., Baylin S. B., Akbani R., Broom B. M., Chen K., Motter T. C., Nguyen K., Weinstein J. N., Zhang N., Ferguson M. L., Adams C., Black A., Bowen J., Gastier-Foster J., Grossman T., Lichtenberg T., Wise L., Davidsen T., Demchok J. A., Shaw K. R., Sheth M., Sofia H. J., Yang L., Downing J. R., Eley G., Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013). - PMC - PubMed
-
- Mardis E. R., Ding L., Dooling D. J., Larson D. E., McLellan M. D., Chen K., Koboldt D. C., Fulton R. S., Delehaunty K. D., McGrath S. D., Fulton L. A., Locke D. P., Magrini V. J., Abbott R. M., Vickery T. L., Reed J. S., Robinson J. S., Wylie T., Smith S. M., Carmichael L., Eldred J. M., Harris C. C., Walker J., Peck J. B., Du F., Dukes A. F., Sanderson G. E., Brummett A. M., Clark E., McMichael J. F., Meyer R. J., Schindler J. K., Pohl C. S., Wallis J. W., Shi X., Lin L., Schmidt H., Tang Y., Haipek C., Wiechert M. E., Ivy J. V., Kalicki J., Elliott G., Ries R. E., Payton J. E., Westervelt P., Tomasson M. H., Watson M. A., Baty J., Heath S., Shannon W. D., Nagarajan R., Link D. C., Walter M. J., Graubert T. A., DiPersio J. F., Wilson R. K., Ley T. J., Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009). - PMC - PubMed
-
- Dang L., White D. W., Gross S., Bennett B. D., Bittinger M. A., Driggers E. M., Fantin V. R., Jang H. G., Jin S., Keenan M. C., Marks K. M., Prins R. M., Ward P. S., Yen K. E., Liau L. M., Rabinowitz J. D., Cantley L. C., Thompson C. B., Vander Heiden M. G., Su S. M., Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465, 966 (2010). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

