Placental senescence pathophysiology is shared between peripartum cardiomyopathy and preeclampsia in mouse and human
- PMID: 38630848
- PMCID: PMC11331492
- DOI: 10.1126/scitranslmed.adi0077
Placental senescence pathophysiology is shared between peripartum cardiomyopathy and preeclampsia in mouse and human
Abstract
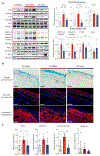
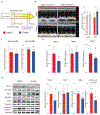
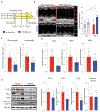
Peripartum cardiomyopathy (PPCM) is an idiopathic form of pregnancy-induced heart failure associated with preeclampsia. Circulating factors in late pregnancy are thought to contribute to both diseases, suggesting a common underlying pathophysiological process. However, what drives this process remains unclear. Using serum proteomics, we identified the senescence-associated secretory phenotype (SASP), a marker of cellular senescence associated with biological aging, as the most highly up-regulated pathway in young women with PPCM or preeclampsia. Placentas from women with preeclampsia displayed multiple markers of amplified senescence and tissue aging, as well as overall increased gene expression of 28 circulating proteins that contributed to SASP pathway enrichment in serum samples from patients with preeclampsia or PPCM. The most highly expressed placental SASP factor, activin A, was associated with cardiac dysfunction or heart failure severity in women with preeclampsia or PPCM. In a murine model of PPCM induced by cardiomyocyte-specific deletion of the gene encoding peroxisome proliferator-activated receptor γ coactivator-1α, inhibiting activin A signaling in the early postpartum period with a monoclonal antibody to the activin type II receptor improved heart function. In addition, attenuating placental senescence with the senolytic compound fisetin in late pregnancy improved cardiac function in these animals. These findings link senescence biology to cardiac dysfunction in pregnancy and help to elucidate the pathogenesis underlying cardiovascular diseases of pregnancy.
Conflict of interest statement
J.D.R. and A.R. are coinventors on patents (WO-2018175460-A1; Methods for Preventing and Treating Heart Disease and 11834508; Method of treating structural and/or functional cardiac abnormalities by administering an anti-ActRII receptor antibody). J.D.R. reports research support from Amgen, Keros, and Genentech. A.R. reports consulting fees from Keros and Versanis as well as serving as a scientific cofounder and equity owner of Thryv Therapeutics. M.C.H. reports research support from Genentech, consulting fees from CRISPR Therapeutics, and advisory board service for Miga Health. S.R. reports serving as a consultant to Roche and Thermo Fisher Scientific and has received funding from Roche and Siemens for studies related to the use of angiogenic factors in pregnancy, which is unrelated to the present work. J.B. reports research support from Zoll, CVRx, Abiomed, Norgine, and Roche and honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, Corvia, Norgine, Edwards, and Roche. P.T.E. reports research support from Bayer AG, Novo-Nordisk, Bristol Myers Squibb and Pfizer and has served on advisory boards or consulted for Bayer AG and MyoKardia. K.J.G. has served as a consultant to Illumina, Aetion, Roche, and BillionToOne. S.A.K. reports research support from Thermo Fisher Scientific, Roche, Siemens, and Beckman Coulter and serves as a consultant for Thermo Fisher Scientific, Roche, and Siemens. S.A.K. has financial interest in Aggamin Pharmaceuticals and Comanche Biopharma and has multiple patents on angiogenic biomarkers that have been outlicensed to multiple companies. All reported research support and consulting are unrelated to this work.
Figures
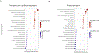


References
-
- Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L, Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2, e323–e333 (2014). - PubMed
-
- Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS, American Heart Association Council on Clinical Cardiology, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular and Stroke Nursing; and Stroke Council, Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation 141, e884–e903 (2020). - PubMed
-
- Hoes MF, Arany Z, Bauersachs J, Hilfiker-Kleiner D, Petrie MC, Sliwa K, van der Meer P, Pathophysiology and risk factors of peripartum cardiomyopathy. Nat. Rev. Cardiol 19, 555–565 (2022). - PubMed
-
- Demakis JG, Rahimtoola SH, Peripartum cardiomyopathy. Circulation 44, 964–968 (1971). - PubMed
-
- Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H, A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128, 589–600 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL170058/HL/NHLBI NIH HHS/United States
- K08 HL166687/HL/NHLBI NIH HHS/United States
- R03 HL162756/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- R37 AG013925/AG/NIA NIH HHS/United States
- R01 HL163234/HL/NHLBI NIH HHS/United States
- R56 HL157579/HL/NHLBI NIH HHS/United States
- R35 HL155318/HL/NHLBI NIH HHS/United States
- RC1 HL102429/HL/NHLBI NIH HHS/United States
- R01 HL126797/HL/NHLBI NIH HHS/United States
- R33 AG061456/AG/NIA NIH HHS/United States
- R01 HL152446/HL/NHLBI NIH HHS/United States
- R01 HL139731/HL/NHLBI NIH HHS/United States
- R01 AG061034/AG/NIA NIH HHS/United States
- T32 HL007208/HL/NHLBI NIH HHS/United States
- R01 HL148191/HL/NHLBI NIH HHS/United States
- K01 AG080077/AG/NIA NIH HHS/United States
- K76 AG064328/AG/NIA NIH HHS/United States
- K08 HL146963/HL/NHLBI NIH HHS/United States
- R01 HL157635/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

