Phase II, Single-Arm Trial of Induction and Concurrent Vismodegib With Curative-Intent Radiation Therapy for Locally Advanced, Unresectable Basal Cell Carcinoma
- PMID: 38630954
- PMCID: PMC11479655
- DOI: 10.1200/JCO.23.01708
Phase II, Single-Arm Trial of Induction and Concurrent Vismodegib With Curative-Intent Radiation Therapy for Locally Advanced, Unresectable Basal Cell Carcinoma
Abstract
Purpose: Locally advanced, unresectable basal cell carcinoma (LA BCC) can be treated with radiation therapy (RT), but locoregional control (LRC) rates are unsatisfactory. Vismodegib is a hedgehog pathway inhibitor (HPI) active in BCC that may radiosensitize BCC. We evaluated the combination of vismodegib and RT for patients with LA BCC.
Methods: In this multicenter, single-arm, phase II study, patients with unresectable LA BCC received 12 weeks of induction vismodegib, followed by 7 weeks of concurrent vismodegib and RT. The primary end point was LRC rate at 1 year after the end of treatment. Secondary end points included objective response, progression-free survival (PFS), overall survival (OS), safety, and patient-reported quality of life (PRQOL).
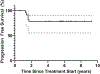
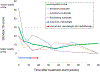
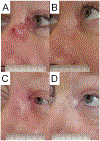
Results: Twenty-four patients received vismodegib; five were unable to complete 12 weeks of induction therapy. LRC was achieved in 91% (95% CI, 68 to 98) of patients at 1 year. The response rate was 63% (95% CI, 38 to 84) after induction vismodegib and 83% (95% CI, 59 to 96) after concurrent vismodegib and RT. With a median follow-up of 5.7 years, 1-year PFS and OS rates were 100% and 96%, and at 5 years PFS and OS rates were 78% and 83%, respectively. Distant metastasis or BCC-related death has not been observed. The most frequent treatment-related adverse events (AEs) were dysgeusia, fatigue, and myalgias occurring in 83%, 75%, and 75% of patients. No grade 4 to 5 treatment-related AEs occurred. PRQOL demonstrated clinically meaningful improvements in all subscales, with emotions and functioning improvements persisting for a year after the end of treatment.
Conclusion: In patients with unresectable LA BCC, the combination of vismodegib and RT yielded high rates of LRC and PFS and durable improvements in PRQOL.
Figures


References
-
- Likhacheva A, Awan M, Barker CA, Bhatnagar A, Bradfield L, Brady MS, Buzurovic I, Geiger JL, Parvathaneni U, Zaky S, Devlin PM.Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract Radiat Oncol. 2020. Jan-Feb;10(1):8–20. doi: 10.1016/j.prro.2019.10.014. Epub 2019 Dec 9. - DOI - PubMed
-
- Wilder RB, Shimm DS, Kittelson JM, Rogoff EE, Cassady JR. Recurrent basal cell carcinoma treated with radiation therapy. Arch Dermatol. 1991. Nov;127(11):1668–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

