Integrating Biomarkers From Virtual Reality and Magnetic Resonance Imaging for the Early Detection of Mild Cognitive Impairment Using a Multimodal Learning Approach: Validation Study
- PMID: 38631021
- PMCID: PMC11063880
- DOI: 10.2196/54538
Integrating Biomarkers From Virtual Reality and Magnetic Resonance Imaging for the Early Detection of Mild Cognitive Impairment Using a Multimodal Learning Approach: Validation Study
Abstract
Background: Early detection of mild cognitive impairment (MCI), a transitional stage between normal aging and Alzheimer disease, is crucial for preventing the progression of dementia. Virtual reality (VR) biomarkers have proven to be effective in capturing behaviors associated with subtle deficits in instrumental activities of daily living, such as challenges in using a food-ordering kiosk, for early detection of MCI. On the other hand, magnetic resonance imaging (MRI) biomarkers have demonstrated their efficacy in quantifying observable structural brain changes that can aid in early MCI detection. Nevertheless, the relationship between VR-derived and MRI biomarkers remains an open question. In this context, we explored the integration of VR-derived and MRI biomarkers to enhance early MCI detection through a multimodal learning approach.
Objective: We aimed to evaluate and compare the efficacy of VR-derived and MRI biomarkers in the classification of MCI while also examining the strengths and weaknesses of each approach. Furthermore, we focused on improving early MCI detection by leveraging multimodal learning to integrate VR-derived and MRI biomarkers.
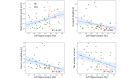
Methods: The study encompassed a total of 54 participants, comprising 22 (41%) healthy controls and 32 (59%) patients with MCI. Participants completed a virtual kiosk test to collect 4 VR-derived biomarkers (hand movement speed, scanpath length, time to completion, and the number of errors), and T1-weighted MRI scans were performed to collect 22 MRI biomarkers from both hemispheres. Analyses of covariance were used to compare these biomarkers between healthy controls and patients with MCI, with age considered as a covariate. Subsequently, the biomarkers that exhibited significant differences between the 2 groups were used to train and validate a multimodal learning model aimed at early screening for patients with MCI among healthy controls.
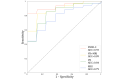
Results: The support vector machine (SVM) using only VR-derived biomarkers achieved a sensitivity of 87.5% and specificity of 90%, whereas the MRI biomarkers showed a sensitivity of 90.9% and specificity of 71.4%. Moreover, a correlation analysis revealed a significant association between MRI-observed brain atrophy and impaired performance in instrumental activities of daily living in the VR environment. Notably, the integration of both VR-derived and MRI biomarkers into a multimodal SVM model yielded superior results compared to unimodal SVM models, achieving higher accuracy (94.4%), sensitivity (100%), specificity (90.9%), precision (87.5%), and F1-score (93.3%).
Conclusions: The results indicate that VR-derived biomarkers, characterized by their high specificity, can be valuable as a robust, early screening tool for MCI in a broader older adult population. On the other hand, MRI biomarkers, known for their high sensitivity, excel at confirming the presence of MCI. Moreover, the multimodal learning approach introduced in our study provides valuable insights into the improvement of early MCI detection by integrating a diverse set of biomarkers.
Keywords: MRI; VR; early detection; eye movement; hand movement; magnetic resonance imaging; mild cognitive impairment; multimodal learning; virtual reality.
©Bogyeom Park, Yuwon Kim, Jinseok Park, Hojin Choi, Seong-Eun Kim, Hokyoung Ryu, Kyoungwon Seo. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 17.04.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





Similar articles
-
Digital Marker for Early Screening of Mild Cognitive Impairment Through Hand and Eye Movement Analysis in Virtual Reality Using Machine Learning: First Validation Study.J Med Internet Res. 2023 Oct 20;25:e48093. doi: 10.2196/48093. J Med Internet Res. 2023. PMID: 37862101 Free PMC article.
-
Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment.Cochrane Database Syst Rev. 2020 Mar 2;3(3):CD009628. doi: 10.1002/14651858.CD009628.pub2. Cochrane Database Syst Rev. 2020. PMID: 32119112 Free PMC article.
-
Anticipatory reaching motor behavior characterizes patients within the Alzheimer's disease continuum in a virtual reality environment.Alzheimers Res Ther. 2025 Apr 9;17(1):78. doi: 10.1186/s13195-025-01726-6. Alzheimers Res Ther. 2025. PMID: 40200361 Free PMC article.
-
Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation.Brain. 2019 Jun 1;142(6):1751-1766. doi: 10.1093/brain/awz116. Brain. 2019. PMID: 31121601 Free PMC article.
-
Efficacy and Moderators of Virtual Reality for Cognitive Training in People with Dementia and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis.J Alzheimers Dis. 2022;88(4):1341-1370. doi: 10.3233/JAD-210672. J Alzheimers Dis. 2022. PMID: 35811514
Cited by
-
A Review of Recent Advances in Cognitive-Motor Dual-Tasking for Parkinson's Disease Rehabilitation.Sensors (Basel). 2024 Sep 30;24(19):6353. doi: 10.3390/s24196353. Sensors (Basel). 2024. PMID: 39409390 Free PMC article. Review.
-
Exploring the Relationship between Behavioral and Neurological Impairments Due to Mild Cognitive Impairment: Correlation Study between Virtual Kiosk Test and EEG-SSVEP.Sensors (Basel). 2024 May 30;24(11):3543. doi: 10.3390/s24113543. Sensors (Basel). 2024. PMID: 38894334 Free PMC article.
-
Using Virtual Reality to Improve Outcomes Related to Quality of Life Among Older Adults With Serious Illnesses: Systematic Review of Randomized Controlled Trials.J Med Internet Res. 2025 Feb 26;27:e54452. doi: 10.2196/54452. J Med Internet Res. 2025. PMID: 40009834 Free PMC article.
-
Alzheimer's disease digital biomarkers multidimensional landscape and AI model scoping review.NPJ Digit Med. 2025 Jun 16;8(1):366. doi: 10.1038/s41746-025-01640-z. NPJ Digit Med. 2025. PMID: 40523935 Free PMC article.
-
Detection of mild cognitive impairment using a virtual reality-based stroop task: a cross-sectional study of embodied behavioral markers.J Neuroeng Rehabil. 2025 Aug 9;22(1):176. doi: 10.1186/s12984-025-01714-9. J Neuroeng Rehabil. 2025. PMID: 40783531 Free PMC article.
References
-
- Opwonya J, Doan DN, Kim SG, Kim JI, Ku B, Kim S, Park S, Kim JU. Saccadic eye movement in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neuropsychol Rev. 2022 Jun 06;32(2):193–227. doi: 10.1007/s11065-021-09495-3. https://europepmc.org/abstract/MED/33959887 10.1007/s11065-021-09495-3 - DOI - PMC - PubMed
-
- Rawtaer I, Mahendran R, Kua EH, Tan HP, Tan HX, Lee TS, Ng TP. Early detection of mild cognitive impairment with in-home sensors to monitor behavior patterns in community-dwelling senior citizens in Singapore: cross-sectional feasibility study. J Med Internet Res. 2020 May 05;22(5):e16854. doi: 10.2196/16854. https://www.jmir.org/2020/5/e16854/ v22i5e16854 - DOI - PMC - PubMed
-
- Ma J, Zhang J, Wang Z. Multimodality Alzheimer's disease analysis in deep Riemannian manifold. Inf Process Manage. 2022 Jul;59(4):102965. doi: 10.1016/j.ipm.2022.102965. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

