Upregulation of coagulation factor V by glucocorticoid in the preovulatory follicles of zebrafish
- PMID: 38631601
- PMCID: PMC11140551
- DOI: 10.1016/j.jsbmb.2024.106521
Upregulation of coagulation factor V by glucocorticoid in the preovulatory follicles of zebrafish
Abstract
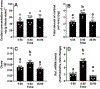
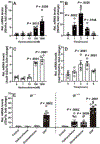
Increased cortisol levels in the preovulatory follicular fluid suggests a role of glucocorticoid in human ovulation. However, the mechanisms through which cortisol regulates the ovulatory process remain poorly understood. In this study, we examined the upregulation of f5 mRNA by glucocorticoid and its receptor (Gr) in the preovulatory follicles of zebrafish. Our findings demonstrate a significant increase in 11β-hydroxysteroid dehydrogenase type 2 (hsd11b2), a cortisol response gene, in preovulatory follicles. Additionally, hydrocortisone exerts a dose- and time-dependent upregulation of f5 mRNA in these follicles. Importantly, this stimulatory effect is Gr-dependent, as it was completely abolished in gr-/- mutants. Furthermore, site-directed mutagenesis identified a glucocorticoid response element (GRE) in the promoter of zebrafish f5. Interestingly, successive incubation of hydrocortisone and the native ovulation-inducing steroid, progestin (17α,20β-dihydroxy-4-pregnen-3-one, DHP), further enhanced f5 expression in preovulatory follicles. Overall, our results indicate that the dramatic increase of f5 expression in preovulatory follicles is partially attributable to the regulation of glucocorticoid and Gr.
Keywords: Coagulation factor; Glucocorticoid; Glucocorticoid receptor; Ovarian follicles.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures
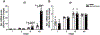

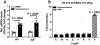

Similar articles
-
11β-Hydroxysteroid dehydrogenase type 2 may mediate the stress-specific effects of cortisol on brain cell proliferation in adult zebrafish (Danio rerio).J Exp Biol. 2024 Aug 15;227(16):jeb248020. doi: 10.1242/jeb.248020. Epub 2024 Aug 26. J Exp Biol. 2024. PMID: 39092490 Free PMC article.
-
The glucocorticoid receptor is affected by its target ZBTB16 in a dissociated manner.J Endocrinol. 2025 Jul 4;266(1):e240283. doi: 10.1530/JOE-24-0283. Print 2025 Jul 1. J Endocrinol. 2025. PMID: 40540650 Free PMC article.
-
Gene expression of 11β-HSD and glucocorticoid receptor in the bovine (Bos taurus) follicle during follicular maturation and atresia: the role of follicular stimulating hormone.J Reprod Dev. 2010 Dec;56(6):616-22. doi: 10.1262/jrd.10-019k. Epub 2010 Aug 11. J Reprod Dev. 2010. PMID: 20710123
-
Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia.Cochrane Database Syst Rev. 2017 Nov 6;11(11):CD008727. doi: 10.1002/14651858.CD008727.pub4. Cochrane Database Syst Rev. 2017. PMID: 29106702 Free PMC article.
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
References
-
- Hillier SG, Tetsuka M. An anti-inflammatory role for glucocorticoids in the ovaries? J Reprod Immunol. 1998;39(1–2):21–27. - PubMed
-
- Caimari F, Valassi E, Garbayo P, Steffensen C, Santos A, Corcoy R, Webb SM. Cushing’s syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine. 2017;55(2):555–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

