Efficacy of LCMV-based cancer immunotherapies is unleashed by intratumoral injections of polyI:C
- PMID: 38631714
- PMCID: PMC11029445
- DOI: 10.1136/jitc-2023-008287
Efficacy of LCMV-based cancer immunotherapies is unleashed by intratumoral injections of polyI:C
Abstract
Background: Lymphocytic choriomeningitis virus (LCMV) belongs to the Arenavirus family known for inducing strong cytotoxic T-cell responses in both mice and humans. LCMV has been engineered for the development of cancer immunotherapies, currently undergoing evaluation in phase I/II clinical trials. Initial findings have demonstrated safety and an exceptional ability to activate and expand tumor-specific T lymphocytes. Combination strategies to maximize the antitumor effectiveness of LCMV-based immunotherapies are being explored.
Methods: We assessed the antitumor therapeutic effects of intratumoral administration of polyinosinic:polycytidylic acid (poly(I:C)) and systemic vaccination using an LCMV-vector expressing non-oncogenic versions of the E6 and E7 antigens of human papillomavirus 16 (artLCMV-E7E6) in a bilateral model engrafting TC-1/A9 cells. This cell line, derived from the parental TC-1, exhibits low MHC class I expression and is highly immune-resistant. The mechanisms underlying the combination's efficacy were investigated through bulk RNA-seq, flow cytometry analyses of the tumor microenvironment, selective depletions using antibodies and clodronate liposomes, Batf3 deficient mice, and in vivo bioluminescence experiments. Finally, we assessed the antitumor effectiveness of the combination of artLCMV-E7E6 with BO-112, a GMP-grade poly(I:C) formulated in polyethyleneimine, currently under evaluation in clinical trials.
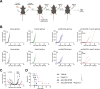
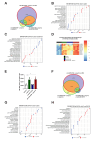
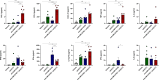
Results: Intratumoral injection of poly(I:C) enhanced the antitumor efficacy of artLCMV-E7E6 in both injected and non-injected tumor lesions. The combined treatment resulted in a significant delay in tumor growth and often complete eradication of several tumor lesions, leading to significantly improved survival compared with monotherapies. While intratumoral administration of poly(I:C) did not impact LCMV vector biodistribution or transgene expression, it significantly modified leucocyte infiltrates within the tumor microenvironment and amplified systemic efficacy through proinflammatory cytokines/chemokines such as CCL3, CCL5, CXCL10, TNF, IFNα, and IL12p70. Upregulation of MHC on tumor cells and a reconfiguration of the gene expression programs related to tumor vasculature, leucocyte migration, and the activation profile of tumor-infiltrating CD8+ T lymphocytes were observed. Indeed, the antitumor effect relied on the functions of CD8+ T lymphocytes and macrophages. The synergistic efficacy of the combination was further confirmed when BO-112 was included.
Conclusion: Intratumoral injection of poly(I:C) sensitizes MHClow tumors to the antitumor effects of artLCMV-E7E6, resulting in a potent therapeutic synergy.
Keywords: Adjuvants, Immunologic; Drug Evaluation, Preclinical; Immunogenicity, Vaccine; Immunotherapy; Vaccination.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PB received research funding from Hookipa Pharma. IM reports receiving commercial research grants from AstraZeneca, BMS, Highlight Therapeutics, Alligator, Pfizer Genmab and Roche; has received speakers bureau honoraria from MSD; and is a consultant or advisory board member for BMS, Roche, AstraZeneca, Genmab, Pharmamar, F-Star, Bioncotech, Bayer, Numab, Pieris, Gossamer, Alligator and Merck Serono. MA declares receiving a commercial research grant from Highlight Therapeutics. TS, KKO, and HL are employees of Hookipa Pharma. MQ is an employee of Highlight Therapeutics. The rest of the authors have no conflict of interest to declare.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
