Restrictive use of Restraints and Delirium Duration in the Intensive Care Unit (R2D2-ICU): protocol for a French multicentre parallel-group open-label randomised controlled trial
- PMID: 38631841
- PMCID: PMC11029382
- DOI: 10.1136/bmjopen-2023-083414
Restrictive use of Restraints and Delirium Duration in the Intensive Care Unit (R2D2-ICU): protocol for a French multicentre parallel-group open-label randomised controlled trial
Abstract
Introduction: Physical restraint (PR) is prescribed in patients receiving invasive mechanical ventilation in the intensive care unit (ICU) to avoid unplanned removal of medical devices. However, it is associated with an increased risk of delirium. We hypothesise that a restrictive use of PR, as compared with a systematic use, could reduce the duration of delirium in ICU patients receiving invasive mechanical ventilation.
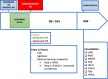
Methods and analysis: The Restrictive use of Restraints and Delirium Duration in ICU (R2D2-ICU) study is a national multicentric, parallel-group, randomised (1:1) open-label, controlled, superiority trial, which will be conducted in 10 ICUs. A total of 422 adult patients requiring invasive mechanical ventilation for an expected duration of at least 48 hours and eligible for prescription of PR will be randomly allocated within 6 hours from intubation to either the restrictive PR use group or the systematic PR use group, until day 14, ICU discharge or death, whichever comes first. In both groups, PR will consist of the use of wrist straps. The primary endpoint will be delirium or coma-free days, defined as the number of days spent alive in the ICU without coma or delirium within the first 14 days after randomisation. Delirium will be assessed using the Confusion Assessment Method-ICU twice daily. Key secondary endpoints will encompass agitation episodes, opioid, propofol, benzodiazepine and antipsychotic drug exposure during the 14-day intervention period, along with a core outcome set of measures evaluated 90 days postrandomisation.
Ethics and dissemination: The R2D2-ICU study has been approved by the Comité de Protection des Personnes (CPP) ILE DE FRANCE III-PARIS (CPP19.09.06.37521) on June 10th, 2019). Participant recruitment started on 25 January 2021. Results will be published in international peer-reviewed medical journals and presented at conferences.
Trial registration number: NCT04273360.
Keywords: Delirium & cognitive disorders; INTENSIVE & CRITICAL CARE; Patient Reported Outcome Measures.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RS received grants from the French Ministry of Health and from LB.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
