Frataxin deficiency shifts metabolism to promote reactive microglia via glucose catabolism
- PMID: 38631900
- PMCID: PMC11024345
- DOI: 10.26508/lsa.202402609
Frataxin deficiency shifts metabolism to promote reactive microglia via glucose catabolism
Abstract
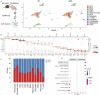
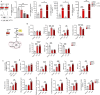
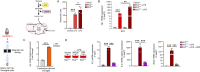
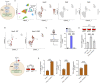
Immunometabolism investigates the intricate relationship between the immune system and cellular metabolism. This study delves into the consequences of mitochondrial frataxin (FXN) depletion, the primary cause of Friedreich's ataxia (FRDA), a debilitating neurodegenerative condition characterized by impaired coordination and muscle control. By using single-cell RNA sequencing, we have identified distinct cellular clusters within the cerebellum of an FRDA mouse model, emphasizing a significant loss in the homeostatic response of microglial cells lacking FXN. Remarkably, these microglia deficient in FXN display heightened reactive responses to inflammatory stimuli. Furthermore, our metabolomic analyses reveal a shift towards glycolysis and itaconate production in these cells. Remarkably, treatment with butyrate counteracts these immunometabolic changes, triggering an antioxidant response via the itaconate-Nrf2-GSH pathways and suppressing the expression of inflammatory genes. Furthermore, we identify Hcar2 (GPR109A) as a mediator involved in restoring the homeostasis of microglia without FXN. Motor function tests conducted on FRDA mice underscore the neuroprotective attributes of butyrate supplementation, enhancing neuromotor performance. In conclusion, our findings elucidate the role of disrupted homeostatic function in cerebellar microglia in the pathogenesis of FRDA. Moreover, they underscore the potential of butyrate to mitigate inflammatory gene expression, correct metabolic imbalances, and improve neuromotor capabilities in FRDA.
© 2024 Sciarretta et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Al-Mahdawi S, Pinto RM, Varshney D, Lawrence L, Lowrie MB, Hughes S, Webster Z, Blake J, Cooper JM, King R, et al. (2006) GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88: 580–590. 10.1016/j.ygeno.2006.06.015 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
