Autocrine VEGF drives neural stem cell proximity to the adult hippocampus vascular niche
- PMID: 38631901
- PMCID: PMC11024344
- DOI: 10.26508/lsa.202402659
Autocrine VEGF drives neural stem cell proximity to the adult hippocampus vascular niche
Abstract
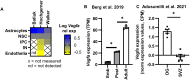
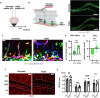
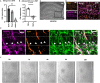
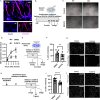
The vasculature is a key component of adult brain neural stem cell (NSC) niches. In the adult mammalian hippocampus, NSCs reside in close contact with a dense capillary network. How this niche is maintained is unclear. We recently found that adult hippocampal NSCs express VEGF, a soluble factor with chemoattractive properties for vascular endothelia. Here, we show that global and NSC-specific VEGF loss led to dissociation of NSCs and their intermediate progenitor daughter cells from local vasculature. Surprisingly, though, we found no changes in local vascular density. Instead, we found that NSC-derived VEGF supports maintenance of gene expression programs in NSCs and their progeny related to cell migration and adhesion. In vitro assays revealed that blockade of VEGF receptor 2 impaired NSC motility and adhesion. Our findings suggest that NSCs maintain their own proximity to vasculature via self-stimulated VEGF signaling that supports their motility towards and/or adhesion to local blood vessels.
© 2024 Dause et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Intracrine VEGF Signaling Is Required for Adult Hippocampal Neural Stem Cell Maintenance and Vascular Proximity.Mol Neurobiol. 2025 Aug;62(8):9604-9624. doi: 10.1007/s12035-025-04861-1. Epub 2025 Mar 25. Mol Neurobiol. 2025. PMID: 40131696 Free PMC article.
-
Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells.Aging Cell. 2011 Jun;10(3):466-82. doi: 10.1111/j.1474-9726.2011.00694.x. Epub 2011 Apr 12. Aging Cell. 2011. PMID: 21385309 Free PMC article.
-
Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans.Cell Rep. 2015 Feb 24;10(7):1158-72. doi: 10.1016/j.celrep.2015.01.049. Epub 2015 Feb 19. Cell Rep. 2015. PMID: 25704818 Free PMC article.
-
The vasculature of neurogenic niches: Properties and function.Cells Dev. 2023 Jun;174:203841. doi: 10.1016/j.cdev.2023.203841. Epub 2023 Apr 14. Cells Dev. 2023. PMID: 37060947 Review.
-
Activity-dependent signaling mechanisms regulating adult hippocampal neural stem cells and their progeny.Neurosci Bull. 2014 Aug;30(4):542-56. doi: 10.1007/s12264-014-1453-5. Epub 2014 Aug 1. Neurosci Bull. 2014. PMID: 25082534 Free PMC article. Review.
Cited by
-
Modern advances in spinal cord regeneration: hydrogel combined with neural stem cells.Front Pharmacol. 2024 Jun 27;15:1419797. doi: 10.3389/fphar.2024.1419797. eCollection 2024. Front Pharmacol. 2024. PMID: 38994202 Free PMC article. Review.
-
Spatiotemporal transcriptomic profiling and modeling of mouse brain at single-cell resolution reveals cell proximity effects of aging and rejuvenation.bioRxiv [Preprint]. 2024 Jul 19:2024.07.16.603809. doi: 10.1101/2024.07.16.603809. bioRxiv. 2024. PMID: 39071282 Free PMC article. Preprint.
-
Brain aging and rejuvenation at single-cell resolution.Neuron. 2025 Jan 8;113(1):82-108. doi: 10.1016/j.neuron.2024.12.007. Neuron. 2025. PMID: 39788089 Review.
-
Intracrine VEGF Signaling Is Required for Adult Hippocampal Neural Stem Cell Maintenance and Vascular Proximity.Mol Neurobiol. 2025 Aug;62(8):9604-9624. doi: 10.1007/s12035-025-04861-1. Epub 2025 Mar 25. Mol Neurobiol. 2025. PMID: 40131696 Free PMC article.
-
Spatial transcriptomic clocks reveal cell proximity effects in brain ageing.Nature. 2025 Feb;638(8049):160-171. doi: 10.1038/s41586-024-08334-8. Epub 2024 Dec 18. Nature. 2025. PMID: 39695234 Free PMC article.
References
-
- Adusumilli VS, Walker TL, Overall RW, Klatt GM, Zeidan SA, Zocher S, Kirova DG, Ntitsias K, Fischer TJ, Sykes AM, et al. (2021) ROS dynamics delineate functional states of hippocampal neural stem cells and link to their activity-dependent exit from quiescence. Cell Stem Cell 28: 300–314.e6. 10.1016/j.stem.2020.10.019 - DOI - PMC - PubMed
-
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman W-H, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093. 10.1093/bioinformatics/btp101 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
