Discovery of Streptomyces species CS-62, a novel producer of the Acinetobacter baumannii selective antibiotic factumycin
- PMID: 38632045
- PMCID: PMC11066910
- DOI: 10.1093/jimb/kuae014
Discovery of Streptomyces species CS-62, a novel producer of the Acinetobacter baumannii selective antibiotic factumycin
Abstract
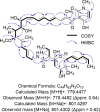
Narrow-spectrum antibiotics are of great interest given their ability to spare the microbiome and decrease widespread antibiotic resistance compared to broad-spectrum antibiotics. Herein, we screened an in-house library of Actinobacteria strains for selective activity against Acinetobacter baumannii and successfully identified Streptomyces sp. CS-62 as a producer of a natural product with this valuable activity. Analysis of the cultures via high-resolution mass spectrometry and tandem mass spectrometry, followed by comparison with molecules in the Natural Product Atlas and the Global Natural Products Social Molecular Networking platform, suggested a novel natural product. Genome mining analysis initially supported the production of a novel kirromycin derivative. Isolation and structure elucidation via mass spectrometry and Nuclear Magnetic Resonance (NMR) analyses revealed that the active natural product was the known natural product factumycin, exposing omissions and errors in the consulted databases. While public databases are generally very useful for avoiding rediscovery of known molecules, rediscovery remains a problem due to public databases either being incomplete or having errors that result in failed dereplication. Overall, the work describes the ongoing problem of dereplication and the continued need for public database curation.
Keywords: Antibiotics; Biosynthetic gene cluster; Dereplication; Natural products.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Corey B. W., Thompson M. G., Hittle L. E., Jacobs A. C., Asafo-Adjei E. A., Huggins W. M., Melander R. J., Melander C., Ernst R. K., Zurawski D. V. (2017). 1,2,4-Triazolidine-3-thiones have specific activity against Acinetobacter baumannii among common nosocomial pathogens. ACS Infectious Diseases, 3(1), 62–71. 10.1021/ACSINFECDIS.6B00133/ASSET/IMAGES/LARGE/ID-2016-00133W_0006.JPEG - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

