An aberrant fused in sarcoma liquid droplet of amyotrophic lateral sclerosis pathological variant, R495X, accelerates liquid-solid phase transition
- PMID: 38632300
- PMCID: PMC11024109
- DOI: 10.1038/s41598-024-59604-4
An aberrant fused in sarcoma liquid droplet of amyotrophic lateral sclerosis pathological variant, R495X, accelerates liquid-solid phase transition
Abstract
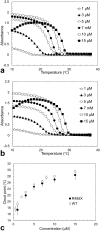
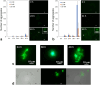
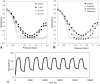
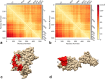
Intracellular aggregation of fused in sarcoma (FUS) is associated with the pathogenesis of familial amyotrophic lateral sclerosis (ALS). Under stress, FUS forms liquid droplets via liquid-liquid phase separation (LLPS). Two types of wild-type FUS LLPS exist in equilibrium: low-pressure LLPS (LP-LLPS) and high-pressure LLPS (HP-LLPS); the former dominates below 2 kbar and the latter over 2 kbar. Although several disease-type FUS variants have been identified, the molecular mechanism underlying accelerated cytoplasmic granule formation in ALS patients remains poorly understood. Herein, we report the reversible formation of the two LLPS states and the irreversible liquid-solid transition, namely droplet aging, of the ALS patient-type FUS variant R495X using fluorescence microscopy and ultraviolet-visible absorption spectroscopy combined with perturbations in pressure and temperature. Liquid-to-solid phase transition was accelerated in the HP-LLPS of R495X than in the wild-type variant; arginine slowed the aging of droplets at atmospheric conditions by inhibiting the formation of HP-LLPS more selectively compared to that of LP-LLPS. Our findings provide new insight into the mechanism by which R495X readily forms cytoplasmic aggregates. Targeting the aberrantly formed liquid droplets (the HP-LLPS state) of proteins with minimal impact on physiological functions could be a novel therapeutic strategy for LLPS-mediated protein diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mechanism underlying liquid-to-solid phase transition in fused in sarcoma liquid droplets.Phys Chem Chem Phys. 2022 Aug 17;24(32):19346-19353. doi: 10.1039/d2cp02171d. Phys Chem Chem Phys. 2022. PMID: 35943083
-
ATP as a Key Modulator of Fused-in-sarcoma Phase Separation and Aggregation: Insights into Amyotrophic Lateral Sclerosis Pathogenesis.J Mol Biol. 2025 Sep 1;437(17):169295. doi: 10.1016/j.jmb.2025.169295. Epub 2025 Jun 16. J Mol Biol. 2025. PMID: 40516596
-
Pressure-Jump Kinetics of Liquid-Liquid Phase Separation: Comparison of Two Different Condensed Phases of the RNA-Binding Protein, Fused in Sarcoma.J Am Chem Soc. 2021 Dec 1;143(47):19697-19702. doi: 10.1021/jacs.1c07571. Epub 2021 Nov 17. J Am Chem Soc. 2021. PMID: 34787417
-
Role and therapeutic potential of liquid-liquid phase separation in amyotrophic lateral sclerosis.J Mol Cell Biol. 2021 Apr 10;13(1):15-28. doi: 10.1093/jmcb/mjaa049. J Mol Cell Biol. 2021. PMID: 32976566 Free PMC article. Review.
-
Molecular Mechanisms of Phase Separation and Amyloidosis of ALS/FTD-linked FUS and TDP-43.Aging Dis. 2024 Oct 1;15(5):2084-2112. doi: 10.14336/AD.2023.1118. Aging Dis. 2024. PMID: 38029395 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

