Selenium catalysis enables negative feedback organic oscillators
- PMID: 38632338
- PMCID: PMC11024130
- DOI: 10.1038/s41467-024-47714-6
Selenium catalysis enables negative feedback organic oscillators
Abstract
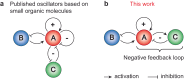
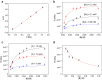
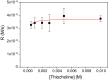
The construction of materials regulated by chemical reaction networks requires regulatory motifs that can be stacked together into systems with desired properties. Multiple autocatalytic reactions producing thiols are known. However, negative feedback loop motifs are unavailable for thiol chemistry. Here, we develop a negative feedback loop based on the selenocarbonates. In this system, thiols induce the release of aromatic selenols that catalyze the oxidation of thiols by organic peroxides. This negative feedback loop has two important features. First, catalytic oxidation of thiols follows Michaelis-Menten-like kinetics, thus increasing nonlinearity for the negative feedback. Second, the strength of the negative feedback can be tuned by varying substituents in selenocarbonates. When combined with the autocatalytic production of thiols in a flow reactor, this negative feedback loop induces sustained oscillations. The availability of this negative feedback motif enables the future construction of oscillatory, homeostatic, adaptive, and other regulatory circuits in life-inspired systems and materials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Autocatalytic, bistable, oscillatory networks of biologically relevant organic reactions.Nature. 2016 Sep 29;537(7622):656-60. doi: 10.1038/nature19776. Nature. 2016. PMID: 27680939
-
Positive feedback promotes oscillations in negative feedback loops.PLoS One. 2014 Aug 15;9(8):e104761. doi: 10.1371/journal.pone.0104761. eCollection 2014. PLoS One. 2014. PMID: 25126951 Free PMC article.
-
Long negative feedback loop enhances period tunability of biological oscillators.J Theor Biol. 2018 Mar 7;440:21-31. doi: 10.1016/j.jtbi.2017.12.014. Epub 2017 Dec 15. J Theor Biol. 2018. PMID: 29253507
-
Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite.Subcell Biochem. 2007;44:83-113. doi: 10.1007/978-1-4020-6051-9_5. Subcell Biochem. 2007. PMID: 18084891 Review.
-
Oscillatory enzyme reactions and Michaelis-Menten kinetics.FEBS Lett. 2013 Sep 2;587(17):2778-84. doi: 10.1016/j.febslet.2013.07.031. Epub 2013 Jul 23. FEBS Lett. 2013. PMID: 23892075 Review.
Cited by
-
Feedback driven autonomous cycles of assembly and disassembly from minimal building blocks.Nat Commun. 2024 Nov 18;15(1):9980. doi: 10.1038/s41467-024-54197-y. Nat Commun. 2024. PMID: 39557837 Free PMC article.
-
Autonomous Dynamic Control of Crown Ether Cargo Release from [2]Rotaxane Carriers in a Piperidine Oscillator.J Am Chem Soc. 2025 Jul 2;147(26):22883-22891. doi: 10.1021/jacs.5c05460. Epub 2025 Jun 17. J Am Chem Soc. 2025. PMID: 40528382 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

