Tumor promoting effect of spheroids in an orthotopic prostate cancer mouse model
- PMID: 38632341
- PMCID: PMC11024136
- DOI: 10.1038/s41598-024-59052-0
Tumor promoting effect of spheroids in an orthotopic prostate cancer mouse model
Abstract
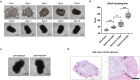
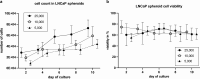
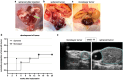
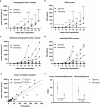
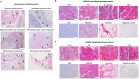
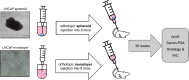
In this study, we aimed to establish a technique for intraprostatic implantation of prostate cancer (PCa) spheroids and to identify the impact of three-dimensional organization of PCa cells on tumor progression and metastasis in a representative in vivo model. 40,000 LNCaP cells were implanted into the prostate of immunodeficient SCID mice either as single cells (n = 8) or as preformed 3D spheroids (n = 8). For a follow up of 20 weeks, tumor growth was monitored by serum PSA and high-resolution 3D ultrasonography. Eventually, animals were sacrificed and autopsied. The organ dissects were analyzed for the presence of metastases by histology (H&E) and immunohistochemistry (AMACR, AR, Ki-67, CK5, CK8, E-Cadherin, Vimentin). Solid intraprostatic tumors developed in 50% of mice after spheroid implantation and in 50% of mice after implantation of a single cells. Primary tumors of LNCaP spheroids evolved earlier, exhibiting a shorter tumor doubling time whilst developing larger tumor volumes, which was reflected by a higher immunohistochemical expression of Ki-67 and AR, too. Spheroid tumors established lung and lymph node metastases in 75% of mice, in contrast to 50% of mice after single cell implantation. Our technique enables a variety of studies regarding the influence of the tumor microenvironment on PCa progression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nowossadeck E, Kowalski C, Barnes B. Demografische Alterung und Krebs. Onkologie heute. 2018;3:48–50.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

