Streptomyces umbrella toxin particles block hyphal growth of competing species
- PMID: 38632398
- PMCID: PMC11062931
- DOI: 10.1038/s41586-024-07298-z
Streptomyces umbrella toxin particles block hyphal growth of competing species
Abstract
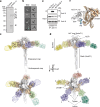
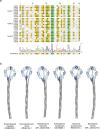
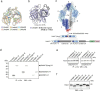
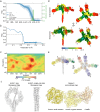
Streptomyces are a genus of ubiquitous soil bacteria from which the majority of clinically utilized antibiotics derive1. The production of these antibacterial molecules reflects the relentless competition Streptomyces engage in with other bacteria, including other Streptomyces species1,2. Here we show that in addition to small-molecule antibiotics, Streptomyces produce and secrete antibacterial protein complexes that feature a large, degenerate repeat-containing polymorphic toxin protein. A cryo-electron microscopy structure of these particles reveals an extended stalk topped by a ringed crown comprising the toxin repeats scaffolding five lectin-tipped spokes, which led us to name them umbrella particles. Streptomyces coelicolor encodes three umbrella particles with distinct toxin and lectin composition. Notably, supernatant containing these toxins specifically and potently inhibits the growth of select Streptomyces species from among a diverse collection of bacteria screened. For one target, Streptomyces griseus, inhibition relies on a single toxin and that intoxication manifests as rapid cessation of vegetative hyphal growth. Our data show that Streptomyces umbrella particles mediate competition among vegetative mycelia of related species, a function distinct from small-molecule antibiotics, which are produced at the onset of reproductive growth and act broadly3,4. Sequence analyses suggest that this role of umbrella particles extends beyond Streptomyces, as we identified umbrella loci in nearly 1,000 species across Actinobacteria.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Similar articles
-
The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp.FEMS Microbiol Lett. 2007 Sep;274(2):311-5. doi: 10.1111/j.1574-6968.2007.00846.x. Epub 2007 Jul 20. FEMS Microbiol Lett. 2007. PMID: 17645525
-
tmRNA of Streptomyces collinus and Streptomyces griseus during the growth and in the presence of antibiotics.Microb Biotechnol. 2009 Jan;2(1):114-22. doi: 10.1111/j.1751-7915.2008.00066.x. Epub 2008 Oct 13. Microb Biotechnol. 2009. PMID: 21261886 Free PMC article.
-
Streptomyces use umbrella toxins to gently compete with kin.Cell Host Microbe. 2024 Jun 12;32(6):779-781. doi: 10.1016/j.chom.2024.05.014. Cell Host Microbe. 2024. PMID: 38870893
-
Molecular regulation of antibiotic biosynthesis in streptomyces.Microbiol Mol Biol Rev. 2013 Mar;77(1):112-43. doi: 10.1128/MMBR.00054-12. Microbiol Mol Biol Rev. 2013. PMID: 23471619 Free PMC article. Review.
-
RNA degradation and the regulation of antibiotic synthesis in Streptomyces.Future Microbiol. 2010 Mar;5(3):419-29. doi: 10.2217/fmb.10.14. Future Microbiol. 2010. PMID: 20210552 Review.
Cited by
-
Global biogeography and projection of antimicrobial toxin genes.Microbiome. 2025 Feb 4;13(1):40. doi: 10.1186/s40168-025-02038-5. Microbiome. 2025. PMID: 39905479 Free PMC article.
-
BetaH proteolysis unleashes an electrostatic-homing antibacterial polymorphic toxin.bioRxiv [Preprint]. 2025 Jul 24:2025.07.24.666436. doi: 10.1101/2025.07.24.666436. bioRxiv. 2025. PMID: 40777288 Free PMC article. Preprint.
-
Regulation to function: A computational approach to specialized metabolism.PLoS Biol. 2025 Jun 13;23(6):e3003205. doi: 10.1371/journal.pbio.3003205. eCollection 2025 Jun. PLoS Biol. 2025. PMID: 40512685 Free PMC article.
-
Rapamycin Plays a Pivotal Role in the Potent Antifungal Activity Exhibited Against Verticillium dahliae by Streptomyces iranensis OE54 and Streptomyces lacaronensis sp. nov. Isolated from Olive Roots.Microorganisms. 2025 Jul 9;13(7):1622. doi: 10.3390/microorganisms13071622. Microorganisms. 2025. PMID: 40732131 Free PMC article.
-
The type II secretion system as an underappreciated and understudied mediator of interbacterial antagonism.Infect Immun. 2024 Aug 13;92(8):e0020724. doi: 10.1128/iai.00207-24. Epub 2024 Jul 9. Infect Immun. 2024. PMID: 38980047 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

