Stepwise activation of a metabotropic glutamate receptor
- PMID: 38632403
- PMCID: PMC11960862
- DOI: 10.1038/s41586-024-07327-x
Stepwise activation of a metabotropic glutamate receptor
Erratum in
-
Author Correction: Stepwise activation of a metabotropic glutamate receptor.Nature. 2024 May;629(8013):E13. doi: 10.1038/s41586-024-07470-5. Nature. 2024. PMID: 38702522 No abstract available.
Abstract
Metabotropic glutamate receptors belong to a family of G protein-coupled receptors that are obligate dimers and possess a large extracellular ligand-binding domain that is linked via a cysteine-rich domain to their 7-transmembrane domain1. Upon activation, these receptors undergo a large conformational change to transmit the ligand binding signal from the extracellular ligand-binding domain to the G protein-coupling 7-transmembrane domain2. In this manuscript, we propose a model for a sequential, multistep activation mechanism of metabotropic glutamate receptor subtype 5. We present a series of structures in lipid nanodiscs, from inactive to fully active, including agonist-bound intermediate states. Further, using bulk and single-molecule fluorescence imaging, we reveal distinct receptor conformations upon allosteric modulator and G protein binding.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
Brian Kobilka is co-founder of and consultant for ConfometRx. The remaining authors declare no competing interest.
Figures



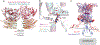
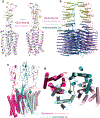


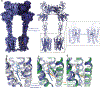







Similar articles
-
Step-wise activation of a Family C GPCR.bioRxiv [Preprint]. 2023 Aug 30:2023.08.29.555158. doi: 10.1101/2023.08.29.555158. bioRxiv. 2023. PMID: 37693614 Free PMC article. Preprint.
-
A photoswitchable positive allosteric modulator to control the activation of the metabotropic glutamate receptor 5 by light.Biochem Pharmacol. 2025 Oct;240:117065. doi: 10.1016/j.bcp.2025.117065. Epub 2025 Jun 24. Biochem Pharmacol. 2025. PMID: 40571212
-
Structural basis of positive allosteric modulation of metabotropic glutamate receptor activation and internalization.Nat Commun. 2024 Aug 1;15(1):6498. doi: 10.1038/s41467-024-50548-x. Nat Commun. 2024. PMID: 39090128 Free PMC article.
-
Negative Allosteric Modulators of Metabotropic Glutamate Receptors Subtype 5 in Addiction: a Therapeutic Window.Int J Neuropsychopharmacol. 2016 Jul 5;19(7):pyw002. doi: 10.1093/ijnp/pyw002. Print 2016 Jul. Int J Neuropsychopharmacol. 2016. PMID: 26802568 Free PMC article.
-
Get Ready to Sharpen Your Tools: A Short Guide to Heterotrimeric G Protein Activity Biosensors.Mol Pharmacol. 2024 Aug 16;106(3):129-144. doi: 10.1124/molpharm.124.000949. Mol Pharmacol. 2024. PMID: 38991745 Review.
Cited by
-
Conformational diversity in class C GPCR positive allosteric modulation.Nat Commun. 2025 Jan 13;16(1):619. doi: 10.1038/s41467-024-55439-9. Nat Commun. 2025. PMID: 39805839 Free PMC article.
-
Pharmacology, Signaling and Therapeutic Potential of Metabotropic Glutamate Receptor 5 Negative Allosteric Modulators.ACS Pharmacol Transl Sci. 2024 Nov 5;7(12):3671-3690. doi: 10.1021/acsptsci.4c00213. eCollection 2024 Dec 13. ACS Pharmacol Transl Sci. 2024. PMID: 39698283 Review.
-
Apo-state structure of the metabotropic glutamate receptor 5 transmembrane domain obtained using a photoswitchable ligand.Protein Sci. 2025 Jul;34(7):e70104. doi: 10.1002/pro.70104. Protein Sci. 2025. PMID: 40521617 Free PMC article.
-
Self-limiting multimerization of α-synuclein on membrane and its implication in Parkinson's diseases.Sci Adv. 2024 Oct 11;10(41):eado4893. doi: 10.1126/sciadv.ado4893. Epub 2024 Oct 9. Sci Adv. 2024. PMID: 39383232 Free PMC article.
-
Efficient screening and discovery of umami peptides in Douchi enhanced by molecular dynamics simulations.Food Chem X. 2024 Oct 29;24:101940. doi: 10.1016/j.fochx.2024.101940. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39559460 Free PMC article.
References
-
- Pin J-P & Bettler B. Organization and functions of mGlu and GABAB receptor complexes. Nature 540, 60–68 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

