Promiscuous G-protein activation by the calcium-sensing receptor
- PMID: 38632411
- PMCID: PMC11844898
- DOI: 10.1038/s41586-024-07331-1
Promiscuous G-protein activation by the calcium-sensing receptor
Abstract
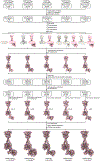
The human calcium-sensing receptor (CaSR) detects fluctuations in the extracellular Ca2+ concentration and maintains Ca2+ homeostasis1,2. It also mediates diverse cellular processes not associated with Ca2+ balance3-5. The functional pleiotropy of CaSR arises in part from its ability to signal through several G-protein subtypes6. We determined structures of CaSR in complex with G proteins from three different subfamilies: Gq, Gi and Gs. We found that the homodimeric CaSR of each complex couples to a single G protein through a common mode. This involves the C-terminal helix of each Gα subunit binding to a shallow pocket that is formed in one CaSR subunit by all three intracellular loops (ICL1-ICL3), an extended transmembrane helix 3 and an ordered C-terminal region. G-protein binding expands the transmembrane dimer interface, which is further stabilized by phospholipid. The restraint imposed by the receptor dimer, in combination with ICL2, enables G-protein activation by facilitating conformational transition of Gα. We identified a single Gα residue that determines Gq and Gs versus Gi selectivity. The length and flexibility of ICL2 allows CaSR to bind all three Gα subtypes, thereby conferring capacity for promiscuous G-protein coupling.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures



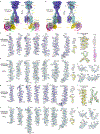

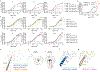





References
-
- Brown EM et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366, 575–580 (1993). - PubMed
-
- Hofer AM & Brown EM Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol 4, 530–538 (2003). - PubMed
-
- Gray E et al. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J. Endocrinol 190, 703–710 (2006). - PubMed
-
- Ruat M & Traiffort E Roles of the calcium sensing receptor in the central nervous system. Best Pract. Res. Clin. Endocrinol. Metab 27, 429–442 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

