Discovery of potent inhibitors of α-synuclein aggregation using structure-based iterative learning
- PMID: 38632492
- PMCID: PMC11062903
- DOI: 10.1038/s41589-024-01580-x
Discovery of potent inhibitors of α-synuclein aggregation using structure-based iterative learning
Abstract
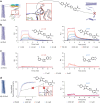
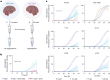
Machine learning methods hold the promise to reduce the costs and the failure rates of conventional drug discovery pipelines. This issue is especially pressing for neurodegenerative diseases, where the development of disease-modifying drugs has been particularly challenging. To address this problem, we describe here a machine learning approach to identify small molecule inhibitors of α-synuclein aggregation, a process implicated in Parkinson's disease and other synucleinopathies. Because the proliferation of α-synuclein aggregates takes place through autocatalytic secondary nucleation, we aim to identify compounds that bind the catalytic sites on the surface of the aggregates. To achieve this goal, we use structure-based machine learning in an iterative manner to first identify and then progressively optimize secondary nucleation inhibitors. Our results demonstrate that this approach leads to the facile identification of compounds two orders of magnitude more potent than previously reported ones.
© 2024. The Author(s).
Conflict of interest statement
R.I.H., M.N., S.C. and P.S. have been consultants of WaveBreak Therapeutics (formerly Wren Therapeutics). R.S. and A.P. have been employees of WaveBreak Therapeutics. M.V. and T.P.J.K. are founders of WaveBreak Therapeutics. WaveBreak Therapeutics is a company that seeks to identify therapeutics for neurodegeneration. The other authors declare no competing interests.
Figures




References
-
- Aarsland D, et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021;7:47. - PubMed
-
- Balestrino R, Schapira AHV. Parkinson disease. Eur. J. Neurol. 2020;27:27–42. - PubMed
-
- Poewe W. Parkinson disease Primer—a true team effort. Nat. Rev. Dis. Prim. 2020;6:31. - PubMed
-
- Savica R, Boeve BF, Mielke MM. When do alpha-synucleinopathies start? An epidemiological timeline: a review. JAMA Neurol. 2018;75:503–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

