DB-1310, an ADC comprised of a novel anti-HER3 antibody conjugated to a DNA topoisomerase I inhibitor, is highly effective for the treatment of HER3-positive solid tumors
- PMID: 38632563
- PMCID: PMC11022355
- DOI: 10.1186/s12967-024-05133-7
DB-1310, an ADC comprised of a novel anti-HER3 antibody conjugated to a DNA topoisomerase I inhibitor, is highly effective for the treatment of HER3-positive solid tumors
Abstract
Background: HER3 (ErbB3), a member of the human epidermal growth factor receptor family, is frequently overexpressed in various cancers. Multiple HER3-targeting antibodies and antibody-drug conjugates (ADCs) were developed for the solid tumor treatment, however none of HER3-targeting agent has been approved for tumor therapy yet. We developed DB-1310, a HER3 ADC composed of a novel humanized anti-HER3 monoclonal antibody covalently linked to a proprietary DNA topoisomerase I inhibitor payload (P1021), and evaluate the efficacy and safety of DB-1310 in preclinical models.
Methods: The binding of DB-1310 to Her3 and other HER families were measured by ELISA and SPR. The competition of binding epitope for DB-1310 and patritumab was tested by FACS. The sensitivity of breast, lung, prostate and colon cancer cell lines to DB-1310 was evaluated by in vitro cell killing assay. In vivo growth inhibition study evaluated the sensitivity of DB-1310 to Her3 + breast, lung, colon and prostate cancer xenograft models. The safety profile was also measured in cynomolgus monkey.
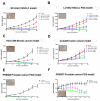
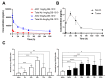
Results: DB-1310 binds HER3 via a novel epitope with high affinity and internalization capacity. In vitro, DB-1310 exhibited cytotoxicity in numerous HER3 + breast, lung, prostate and colon cancer cell lines. In vivo studies in HER3 + HCC1569 breast cancer, NCI-H441 lung cancer and Colo205 colon cancer xenograft models showed DB-1310 to have dose-dependent tumoricidal activity. Tumor suppression was also observed in HER3 + non-small cell lung cancer (NSCLC) and prostate cancer patient-derived xenograft (PDX) models. Moreover, DB-1310 showed stronger tumor growth-inhibitory activity than patritumab deruxtecan (HER3-DXd), which is another HER3 ADC in clinical development at the same dose. The tumor-suppressive activity of DB-1310 synergized with that of EGFR tyrosine kinase inhibitor, osimertinib, and exerted efficacy also in osimertinib-resistant PDX model. The preclinical assessment of safety in cynomolgus monkeys further revealed DB-1310 to have a good safety profile with a highest non severely toxic dose (HNSTD) of 45 mg/kg.
Conclusions: These finding demonstrated that DB-1310 exerted potent antitumor activities against HER3 + tumors in in vitro and in vivo models, and showed acceptable safety profiles in nonclinical species. Therefore, DB-1310 may be effective for the clinical treatment of HER3 + solid tumors.
Keywords: Antibody-drug conjugate; HER3; Preclinical; Solid tumor therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Inaki K, Shibutani T, Maeda N, Eppenberger-Castori S, Nicolet S, Kaneda Y, et al. Pan-cancer gene expression analysis of tissue microarray using EdgeSeq oncology biomarker panel and a cross-comparison with HER2 and HER3 immunohistochemical analysis. PLoS ONE. 2022;17:e0274140. doi: 10.1371/journal.pone.0274140. - DOI - PMC - PubMed
-
- Jänne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 2022;12:74–89. doi: 10.1158/2159-8290.CD-21-0715. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

