Microbiota-derived acetate attenuates neuroinflammation in rostral ventrolateral medulla of spontaneously hypertensive rats
- PMID: 38632579
- PMCID: PMC11025215
- DOI: 10.1186/s12974-024-03061-3
Microbiota-derived acetate attenuates neuroinflammation in rostral ventrolateral medulla of spontaneously hypertensive rats
Abstract
Background: Increased neuroinflammation in brain regions regulating sympathetic nerves is associated with hypertension. Emerging evidence from both human and animal studies suggests a link between hypertension and gut microbiota, as well as microbiota-derived metabolites short-chain fatty acids (SCFAs). However, the precise mechanisms underlying this gut-brain axis remain unclear.
Methods: The levels of microbiota-derived SCFAs in spontaneously hypertensive rats (SHRs) were determined by gas chromatography-mass spectrometry. To observe the effect of acetate on arterial blood pressure (ABP) in rats, sodium acetate was supplemented via drinking water for continuous 7 days. ABP was recorded by radio telemetry. The inflammatory factors, morphology of microglia and astrocytes in rostral ventrolateral medulla (RVLM) were detected. In addition, blood-brain barrier (BBB) permeability, composition and metabolomics of the gut microbiome, and intestinal pathological manifestations were also measured.
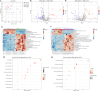
Results: The serum acetate levels in SHRs are lower than in normotensive control rats. Supplementation with acetate reduces ABP, inhibits sympathetic nerve activity in SHRs. Furthermore, acetate suppresses RVLM neuroinflammation in SHRs, increases microglia and astrocyte morphologic complexity, decreases BBB permeability, modulates intestinal flora, increases fecal flora metabolites, and inhibits intestinal fibrosis.
Conclusions: Microbiota-derived acetate exerts antihypertensive effects by modulating microglia and astrocytes and inhibiting neuroinflammation and sympathetic output.
Keywords: Gut microbiota; Hypertension; Neuroinflammation; Short-chain fatty acid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

