Swim bladder-derived biomaterials: structures, compositions, properties, modifications, and biomedical applications
- PMID: 38632585
- PMCID: PMC11022367
- DOI: 10.1186/s12951-024-02449-w
Swim bladder-derived biomaterials: structures, compositions, properties, modifications, and biomedical applications
Abstract
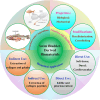
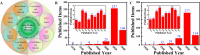
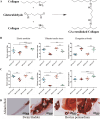
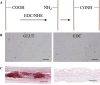
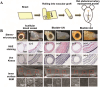
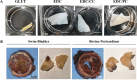
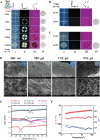
Animal-derived biomaterials have been extensively employed in clinical practice owing to their compositional and structural similarities with those of human tissues and organs, exhibiting good mechanical properties and biocompatibility, and extensive sources. However, there is an associated risk of infection with pathogenic microorganisms after the implantation of tissues from pigs, cattle, and other mammals in humans. Therefore, researchers have begun to explore the development of non-mammalian regenerative biomaterials. Among these is the swim bladder, a fish-derived biomaterial that is rapidly used in various fields of biomedicine because of its high collagen, elastin, and polysaccharide content. However, relevant reviews on the biomedical applications of swim bladders as effective biomaterials are lacking. Therefore, based on our previous research and in-depth understanding of this field, this review describes the structures and compositions, properties, and modifications of the swim bladder, with their direct (including soft tissue repair, dural repair, cardiovascular repair, and edible and pharmaceutical fish maw) and indirect applications (including extracted collagen peptides with smaller molecular weights, and collagen or gelatin with higher molecular weights used for hydrogels, and biological adhesives or glues) in the field of biomedicine in recent years. This review provides insights into the use of swim bladders as source of biomaterial; hence, it can aid biomedicine scholars by providing directions for advancements in this field.
Keywords: Biological adhesive; Cardiovascular repair; Hydrogel; Swim bladder; Tissue repair.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Immunogenicity assessment of swim bladder-derived biomaterials.Biomater Sci. 2023 Apr 11;11(8):2738-2749. doi: 10.1039/d2bm01419j. Biomater Sci. 2023. PMID: 36807688
-
Biomaterial functionalization with triple-helical peptides for tissue engineering.Acta Biomater. 2022 Aug;148:1-21. doi: 10.1016/j.actbio.2022.06.003. Epub 2022 Jun 5. Acta Biomater. 2022. PMID: 35675889 Review.
-
Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications.Tissue Eng Part B Rev. 2020 Apr;26(2):164-180. doi: 10.1089/ten.TEB.2019.0256. Epub 2020 Feb 4. Tissue Eng Part B Rev. 2020. PMID: 31910095 Review.
-
A Thermostable Type I Collagen from Swim Bladder of Silver Carp (Hypophthalmichthys molitrix).Mar Drugs. 2023 Apr 28;21(5):280. doi: 10.3390/md21050280. Mar Drugs. 2023. PMID: 37233474 Free PMC article.
-
Fish Gelatin: Current Nutritional, Medicinal, Tissue Repair Applications, and as a Carrier of Drug Delivery.Curr Pharm Des. 2022;28(12):1019-1030. doi: 10.2174/1381612828666220128103725. Curr Pharm Des. 2022. PMID: 35088658 Review.
Cited by
-
Acoustic Transmitted Decellularized Fish Bladder for Tympanic Membrane Regeneration.Research (Wash D C). 2025 Feb 5;8:0596. doi: 10.34133/research.0596. eCollection 2025. Research (Wash D C). 2025. PMID: 39916796 Free PMC article.
-
Influence of Liquid Nitrogen Pre-Freezing and Drying Methods on the Collagen Content, Physical Properties, and Flavor of Fish Swim Bladder.Foods. 2024 Sep 1;13(17):2790. doi: 10.3390/foods13172790. Foods. 2024. PMID: 39272555 Free PMC article.
-
Tissue-engineered tubular substitutions for urinary diversion in a preclinical rabbit model.Front Med (Lausanne). 2025 Jun 26;12:1616977. doi: 10.3389/fmed.2025.1616977. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40641968 Free PMC article.
References
-
- Lei Y, Lan X, He Z, Yin A, Jin W, Hu Q, Wang Y. Multifarious anti-biofouling bioprosthetic heart valve materials with the formation of interpenetrating polymer network structures. Mater Des. 2021;206:109803. doi: 10.1016/j.matdes.2021.109803. - DOI
-
- Xu H, Wan H, Zuo W, Sun W, Owens RT, Harper JR, Ayares DL, McQuillan DJ. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-α-(1,3)-galactose and retention of matrix structure. Tissue Eng Part A. 2009;15:1807–1819. doi: 10.1089/ten.tea.2008.0384. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFS0634/Sichuan Science and Technology Program
- 2022NSFSC0599/Sichuan Science and Technology Program
- 2022-GYF-12/Key Research and Development Programs of Luzhou
- 2022-SYF-33/Key Research and Development Programs of Luzhou
- 2022BS02/Talent Introduction Program of The Affiliated Stomatological Hospital of Southwest Medical University
- 2022LJ02/Innovative Leading Talents Program of The Affiliated Stomatological Hospital of Southwest Medical University
- 2022LJ01/Innovative Leading Talents Program of The Affiliated Stomatological Hospital of Southwest Medical University
- 81870746/National Natural Science Foundation of China
- 2022ZYD0082/Special Project for Local Science and Technology Development Guided by the Central Government of Sichuan Province
LinkOut - more resources
Full Text Sources

