Superior protection in a relapsing Plasmodium cynomolgi rhesus macaque model by a chemoprophylaxis with sporozoite immunization regimen with atovaquone-proguanil followed by primaquine
- PMID: 38632607
- PMCID: PMC11022453
- DOI: 10.1186/s12936-024-04933-y
Superior protection in a relapsing Plasmodium cynomolgi rhesus macaque model by a chemoprophylaxis with sporozoite immunization regimen with atovaquone-proguanil followed by primaquine
Abstract
Background: To gain a deeper understanding of protective immunity against relapsing malaria, this study examined sporozoite-specific T cell responses induced by a chemoprophylaxis with sporozoite (CPS) immunization in a relapsing Plasmodium cynomolgi rhesus macaque model.
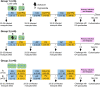
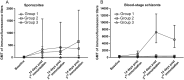
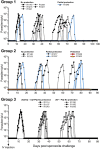
Methods: The animals received three CPS immunizations with P. cynomolgi sporozoites, administered by mosquito bite, while under two anti-malarial drug regimens. Group 1 (n = 6) received artesunate/chloroquine (AS/CQ) followed by a radical cure with CQ plus primaquine (PQ). Group 2 (n = 6) received atovaquone-proguanil (AP) followed by PQ. After the final immunization, the animals were challenged with intravenous injection of 104 P. cynomolgi sporozoites, the dose that induced reliable infection and relapse rate. These animals, along with control animals (n = 6), were monitored for primary infection and subsequent relapses. Immunogenicity blood draws were done after each of the three CPS session, before and after the challenge, with liver, spleen and bone marrow sampling and analysis done after the challenge.
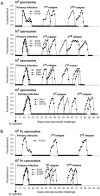
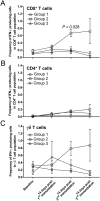
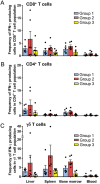
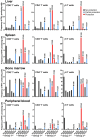
Results: Group 2 animals demonstrated superior protection, with two achieving protection and two experiencing partial protection, while only one animal in group 1 had partial protection. These animals displayed high sporozoite-specific IFN-γ T cell responses in the liver, spleen, and bone marrow after the challenge with one protected animal having the highest frequency of IFN-γ+ CD8+, IFN-γ+ CD4+, and IFN-γ+ γδ T cells in the liver. Partially protected animals also demonstrated a relatively high frequency of IFN-γ+ CD8+, IFN-γ+ CD4+, and IFN-γ+ γδ T cells in the liver. It is important to highlight that the second animal in group 2, which experienced protection, exhibited deficient sporozoite-specific T cell responses in the liver while displaying average to high T cell responses in the spleen and bone marrow.
Conclusions: This research supports the notion that local liver T cell immunity plays a crucial role in defending against liver-stage infection. Nevertheless, there is an instance where protection occurs independently of T cell responses in the liver, suggesting the involvement of the liver's innate immunity. The relapsing P. cynomolgi rhesus macaque model holds promise for informing the development of vaccines against relapsing P. vivax.
Keywords: Atovaquone-proguanil; Chemoprophylaxis with sporozoite immunization; Relapsing Plasmodium cynomolgi rhesus macaque model; Sporozoite-specific T cell responses.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- WHO. World Malaria Report 2023. Geneva, World Health Organization; 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

