IgM anti-GM2 antibodies in patients with multifocal motor neuropathy target Schwann cells and are associated with early onset
- PMID: 38632654
- PMCID: PMC11025174
- DOI: 10.1186/s12974-024-03090-y
IgM anti-GM2 antibodies in patients with multifocal motor neuropathy target Schwann cells and are associated with early onset
Abstract
Background: Multifocal motor neuropathy (MMN) is a rare, chronic immune-mediated polyneuropathy characterized by asymmetric distal limb weakness. An important feature of MMN is the presence of IgM antibodies against gangliosides, in particular GM1 and less often GM2. Antibodies against GM1 bind to motor neurons (MNs) and cause damage through complement activation. The involvement of Schwann cells (SCs), expressing GM1 and GM2, in the pathogenesis of MMN is unknown.
Methods: Combining the data of our 2007 and 2015 combined cross-sectional and follow-up studies in Dutch patients with MMN, we evaluated the presence of IgM antibodies against GM1 and GM2 in serum from 124 patients with MMN and investigated their binding to SCs and complement-activating properties. We also assessed the relation of IgM binding and complement deposition with clinical characteristics.
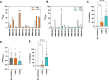
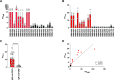
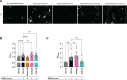
Results: Thirteen out of 124 patients (10%) had a positive ELISA titer for IgM anti-GM2. Age at onset of symptoms was significantly lower in MMN patients with anti-GM2 IgM. IgM binding to SCs correlated with IgM anti-GM2 titers. We found no correlation between IgM anti-GM2 titers and MN binding or with IgM anti-GM1 titers. IgM binding to SCs decreased upon pre-incubation of serum with soluble GM2, but not with soluble GM1. IgM anti-GM2 binding to SCs correlated with complement activation, as reflected by increased C3 fixation on SCs and C5a formation in the supernatant.
Conclusion: Circulating IgM anti-GM2 antibodies define a subgroup of patients with MMN that has an earlier onset of disease. These antibodies probably target SCs specifically and activate complement, similarly as IgM anti-GM1 on MNs. Our data indicate that complement activation by IgM antibodies bound to SCs and MNs underlies MMN pathology.
Keywords: Anti-ganglioside antibodies; Complement; IgM anti-GM2; Multifocal motor neuropathy; Schwann cells.
© 2024. The Author(s).
Conflict of interest statement
KB, EdZ, KD, LMB are employees of the UMC Utrecht on a research service collaboration with argenx BVBA.
JWB reports no disclosures.
EMZ reports no disclosures.
EJNG reports a research grants from the Prinses Beatrix Spierfonds and has received teaching fees from Biogen, all paid to the institution.
BCJ reports grants for research from the GBS/CIDP Foundation International, Prinses Beatrix Spierfonds, Horizon 2020, NIH, Erasmus MC, Annexon, CSL-Behring, Grifols, Hansa Biopharma, Roche and Octapharma. BCJ is chairing the Steering Committee of the International GBS Outcome Study (IGOS), a member of the Advisory Board of Hansa Biopharma and Annexon and of the Global Medical Advisory Board of the GBS-CIDP Foundation International.
RH reports grants from the GBS/CIDP Foundation International, NIH and the T2B collaboration project funded by PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public-private partnerships and co-financing by health foundations that are part of the SGF.
HSG has received research grants from the Prinses Beatrix Spierfonds, speaker fees, and consultancy for Takeda and Quaralis all paid to the institution.
EC reports no disclosures.
JHWL reports no disclosures.
LHvdB received an educational grant from Takeda; serves on the editorial boards of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration; and receives research support from the Netherlands ALS Foundation.
CEH provides consultancy services to argenx BVBA.
WLvdP serves on the scientific advisory board for SAB SMA Europe; provides ad hoc consultancy for argenx BVBA, Biogen, and Novartis genetherapies; is the local PI for the ARDA and ARDA + trials and receives research support from the Prinses Beatrix Spierfonds, Vriendenloterij and Stichting Spieren voor Spieren.
Figures


Similar articles
-
Sensitivity and predictive value of anti-GM1/galactocerebroside IgM antibodies in multifocal motor neuropathy.J Neurol Neurosurg Psychiatry. 2014 Jul;85(7):754-8. doi: 10.1136/jnnp-2013-305755. Epub 2013 Aug 1. J Neurol Neurosurg Psychiatry. 2014. PMID: 23907602
-
Anti-C2 Antibody ARGX-117 Inhibits Complement in a Disease Model for Multifocal Motor Neuropathy.Neurol Neuroimmunol Neuroinflamm. 2021 Nov 10;9(1):e1107. doi: 10.1212/NXI.0000000000001107. Print 2022 Jan. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 34759020 Free PMC article.
-
Childhood-onset multifocal motor neuropathy with IgM antibodies to GM2 and GalNac-GD1a.Brain Dev. 2020 Jan;42(1):88-92. doi: 10.1016/j.braindev.2019.08.013. Epub 2019 Sep 12. Brain Dev. 2020. PMID: 31522790
-
Childhood-Onset Multifocal Motor Neuropathy With Immunoglobulin M Antibodies to Gangliosides GM1 and GM2: A Case Report and Review of the Literature.Pediatr Neurol. 2016 Sep;62:51-7. doi: 10.1016/j.pediatrneurol.2016.03.017. Epub 2016 Apr 21. Pediatr Neurol. 2016. PMID: 27400822 Review.
-
Immune pathogenesis and treatment of multifocal motor neuropathy.J Clin Immunol. 2013 Jan;33 Suppl 1:S38-42. doi: 10.1007/s10875-012-9779-8. Epub 2012 Sep 2. J Clin Immunol. 2013. PMID: 22941513 Review.
Cited by
-
Complement activation by IgM autoantibodies linked to immune-mediated neuropathies depends on C2.Eur J Neurol. 2025 Jan;32(1):e16541. doi: 10.1111/ene.16541. Epub 2024 Nov 15. Eur J Neurol. 2025. PMID: 39545641 Free PMC article.
References
-
- Pestronk A. Multifocal motor neuropathy: diagnosis and treatment. Neurology. 1998;51(6 Suppl 5):S22–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

