Psammaplin A and Its Analogs Attenuate Oxidative Stress in Neuronal Cells through Peroxisome Proliferator-Activated Receptor γ Activation
- PMID: 38632902
- PMCID: PMC11061836
- DOI: 10.1021/acs.jnatprod.4c00153
Psammaplin A and Its Analogs Attenuate Oxidative Stress in Neuronal Cells through Peroxisome Proliferator-Activated Receptor γ Activation
Abstract
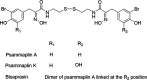
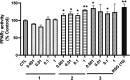
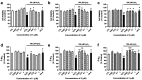
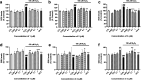
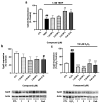
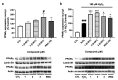
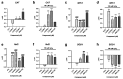
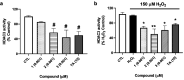
Psammaplins are sulfur containing bromotyrosine alkaloids that have shown antitumor activity through the inhibition of class I histone deacetylases (HDACs). The cytotoxic properties of psammaplin A (1), the parent compound, are related to peroxisome proliferator-activated receptor γ (PPARγ) activation, but the mechanism of action of its analogs psammaplin K (2) and bisaprasin (3) has not been elucidated. In this study, the protective effects against oxidative stress of compounds 1-3, isolated from the sponge Aplysinella rhax, were evaluated in SH-SY5Y cells. The compounds improved cell survival, recovered glutathione (GSH) content, and reduced reactive oxygen species (ROS) release at nanomolar concentrations. Psammaplins restored mitochondrial membrane potential by blocking mitochondrial permeability transition pore opening and reducing cyclophilin D expression. This effect was mediated by the capacity of 1-3 to activate PPARγ, enhancing gene expression of the antioxidant enzymes catalase, nuclear factor E2-related factor 2 (Nrf2), and glutathione peroxidase. Finally, HDAC3 activity was reduced by 1-3 under oxidative stress conditions. This work is the first description of the neuroprotective activity of 1 at low concentrations and the mechanism of action of 2 and 3. Moreover, it links for the first time the previously described effects of 1 in HDAC3 and PPARγ signaling, opening a new research field for the therapeutic potential of this compound family.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Oluwabusola E. T.; Katermeran N. P.; Poh W. H.; Goh T. M. B.; Tan L. T.; Diyaolu O.; Tabudravu J.; Ebel R.; Rice S. A.; Jaspars M. Inhibition of the Quorum Sensing System, Elastase Production and Biofilm Formation in Pseudomonas aeruginosa by Psammaplin A and Bisaprasin. Molecules 2022, 27 (5), 1721. 10.3390/molecules27051721. - DOI - PMC - PubMed
-
- Oluwabusola E. T.; Tabudravu J. N.; Al Maqbali K. S.; Annang F.; Perez-Moreno G.; Reyes F.; Jaspars M. Antiparasitic Activity of Bromotyrosine Alkaloids and New Analogues Isolated from the Fijian Marine Sponge Aplysinella rhax. Chem. Biodivers 2020, 17 (10), e2000335 10.1002/cbdv.202000335. - DOI - PubMed
-
- Kim T. H.; Kim H. S.; Kang Y. J.; Yoon S.; Lee J.; Choi W. S.; Jung J. H.; Kim H. S. Psammaplin A induces Sirtuin 1-dependent autophagic cell death in doxorubicin-resistant MCF-7/adr human breast cancer cells and xenografts. Biochim. Biophys. Acta 2015, 1850 (2), 401–10. 10.1016/j.bbagen.2014.11.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

