Heterochronic shift in gene expression leads to ontogenetic morphological divergence between two closely related polyploid species
- PMID: 38632992
- PMCID: PMC11022054
- DOI: 10.1016/j.isci.2024.109566
Heterochronic shift in gene expression leads to ontogenetic morphological divergence between two closely related polyploid species
Abstract
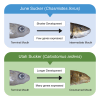
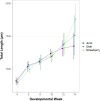
Heterochrony-alteration to the rate or timing of development-is an important mechanism of trait differentiation associated with speciation. Heterochrony may explain the morphological divergence between two polyploid species, June sucker (Chasmistes liorus) and Utah sucker (Catostomus ardens). The larvae of both species have terminal mouths; however, as adults, June sucker and Utah sucker develop subterminal and ventral mouths, respectively. We document a difference in the timing of shape development and a corresponding change in the timing of gene expression, suggesting the distinctive mouth morphology in June suckers may result from paedomorphosis. Specifically, adult June suckers exhibit an intermediate mouth morphology between the larval (terminal) and ancestral (ventral) states. Endemic and sympatric Chasmistes/Catostomus pairs in two other lakes also are morphologically divergent, but genetically similar. These species pairs could have resulted from the differential expression of genes and corresponding divergence in trait development. Paedomorphosis may lead to adaptive diversification in Catostomids.
Keywords: Animal morphology; Evolutionary Developmental Biology; Transcriptomics.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Complete mitochondrial genomes of June sucker and Utah sucker (Chasmistes liorus and Catostomus ardens).Mitochondrial DNA B Resour. 2022 Mar 28;7(3):560-562. doi: 10.1080/23802359.2022.2055984. eCollection 2022. Mitochondrial DNA B Resour. 2022. PMID: 35372694 Free PMC article.
-
Multivariate heritability of shape in June sucker (Chasmistes liorus) and Utah sucker (Catostomus ardens): shape as a functional trait for discriminating closely related species.Dev Genes Evol. 2016 Jun;226(3):197-207. doi: 10.1007/s00427-016-0547-2. Epub 2016 Apr 30. Dev Genes Evol. 2016. PMID: 27138282
-
Morphological and genetic structuring in the Utah Lake sucker complex.Mol Ecol. 2008 Dec;17(24):5189-204. doi: 10.1111/j.1365-294X.2008.03990.x. Epub 2008 Nov 26. Mol Ecol. 2008. PMID: 19067800
-
Time to synchronize our clocks: Connecting developmental mechanisms and evolutionary consequences of heterochrony.J Exp Zool B Mol Dev Evol. 2022 Jan;338(1-2):87-106. doi: 10.1002/jez.b.23103. Epub 2021 Nov 26. J Exp Zool B Mol Dev Evol. 2022. PMID: 34826199 Review.
-
The Times They Are A-Changin': Heterochrony in Plant Development and Evolution.Front Plant Sci. 2018 Sep 18;9:1349. doi: 10.3389/fpls.2018.01349. eCollection 2018. Front Plant Sci. 2018. PMID: 30283473 Free PMC article. Review.
References
-
- Skúlason S., Parsons K.J., Svanbäck R., Räsänen K., Ferguson M.M., Adams C.E., Amundsen P.A., Bartels P., Bean C.W., Boughman J.W., et al. A way forward with eco evo devo: an extended theory of resource polymorphism with postglacial fishes as model systems. Biol. Rev. 2019;94:1786–1808. doi: 10.1111/brv.12534. - DOI - PMC - PubMed
-
- Martynov A., Korshunova T. In: Cryptic Species: Morphological Stasis, Circumscription, and Hidden Diversity. Monro A.K., Mayo S.J., editors. Cambridge University Press; 2022. Multilevel Organismal Diversity in an Ontogenetic Framework as a Solution for the Species Concept; pp. 78–129. - DOI
-
- Martynov A., Lundin K., Korshunova T. Ontogeny, Phylotypic Periods, Paedomorphosis, and Ontogenetic Systematics. Front. Ecol. Evol. 2022;10:806414. doi: 10.3389/fevo.2022.806414. - DOI
-
- Smith K.K. Time's arrow: heterochrony and the evolution of development. Int. J. Dev. Biol. 2003;47:613–621. - PubMed
LinkOut - more resources
Full Text Sources

