Probing the pH-dependency of DC-SIGN/R multivalent lectin-glycan interactions using polyvalent glycan-gold nanoparticles
- PMID: 38633047
- PMCID: PMC11019501
- DOI: 10.1039/d3na01013a
Probing the pH-dependency of DC-SIGN/R multivalent lectin-glycan interactions using polyvalent glycan-gold nanoparticles
Abstract
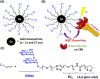
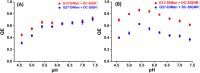
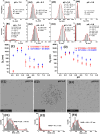
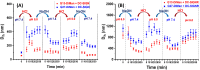
The dendritic cell tetrameric lectin, DC-SIGN, and its closely related endothelial cell lectin, DC-SIGNR (collectively abbreviated as DC-SIGN/R) play a key role in the binding and transmission of deadly viruses, including Ebola, HIV, HCV, and SARS-CoV-2. Their virus binding/release processes involve a gradually acidifying environment following the natural intracellular trafficking pathways. Therefore, understanding DC-SIGN/R's pH-dependent binding properties with glycan ligands is of great importance. We have recently developed densely glycosylated gold nanoparticles (glycan-GNPs) as a powerful new tool for probing DC-SIGN/R multivalent lectin-glycan interaction (MLGI) mechanisms. They can provide not only quantitative MLGI affinities but also important structural information, such as binding site orientation and binding modes. Herein, we further employ the glycan-GNP probes to investigate the pH dependency of DC-SIGN/R MLGI properties. We find that DC-SIGN/R MLGIs exhibit distinct pH dependence over the normal physiological (7.4) to lysosomal (∼4.6) pH range. DC-SIGN binds glycan-GNPs strongly and stably from pH 7.4 to ∼5.8, but the binding is weakened significantly as pH decreases to ≤5.4 and may be fully dissociated at pH 4.6. This behaviour is fully consistent with DC-SIGN's role as an endocytic recycling receptor. In contrast, DC-SIGNR's affinity with glycan-GNPs is enhanced with the decreasing pH from 7.4 to 5.4, peaking at pH 5.4, and then reduced as pH is further lowered. Interestingly, both DC-SIGN/R binding with glycan-GNPs are found to be partially reversible in a pH-dependent manner.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
References
-
- Huskens J., Prins L. J., Haag R. and Ravoo B. J., Multivalency: Concepts, Research and Applications, John Wiley & Sons, 2018
LinkOut - more resources
Full Text Sources
Miscellaneous

