Dual modality intravascular catheter system combining pulse-sampling fluorescence lifetime imaging and polarization-sensitive optical coherence tomography
- PMID: 38633060
- PMCID: PMC11019710
- DOI: 10.1364/BOE.516515
Dual modality intravascular catheter system combining pulse-sampling fluorescence lifetime imaging and polarization-sensitive optical coherence tomography
Abstract
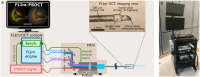
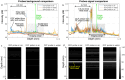
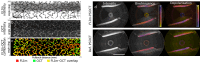
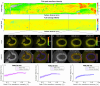
The clinical management of coronary artery disease and the prevention of acute coronary syndromes require knowledge of the underlying atherosclerotic plaque pathobiology. Hybrid imaging modalities capable of comprehensive assessment of biochemical and morphological plaques features can address this need. Here we report the first implementation of an intravascular catheter system combining fluorescence lifetime imaging (FLIm) with polarization-sensitive optical coherence tomography (PSOCT). This system provides multi-scale assessment of plaque structure and composition via high spatial resolution morphology from OCT, polarimetry-derived tissue microstructure, and biochemical composition from FLIm, without requiring any molecular contrast agent. This result was achieved with a low profile (2.7 Fr) double-clad fiber (DCF) catheter and high speed (100 fps B-scan rate, 40 mm/s pullback speed) console. Use of a DCF and broadband rotary junction required extensive optimization to mitigate the reduction in OCT performance originating from additional reflections and multipath artifacts. This challenge was addressed by the development of a broad-band (UV-visible-IR), high return loss (47 dB) rotary junction. We demonstrate in phantoms, ex vivo swine coronary specimens and in vivo swine heart (percutaneous coronary access) that the FLIm-PSOCT catheter system can simultaneously acquire co-registered FLIm data over four distinct spectral bands (380/20 nm, 400/20 nm, 452/45 nm, 540/45 nm) and PSOCT backscattered intensity, birefringence, and depolarization. The unique ability to collect complementary information from tissue (e.g., morphology, extracellular matrix composition, inflammation) with a device suitable for percutaneous coronary intervention offers new opportunities for cardiovascular research and clinical diagnosis.
© 2024 Optica Publishing Group.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures


Similar articles
-
Dual-modality fluorescence lifetime imaging-optical coherence tomography intravascular catheter system with freeform catheter optics.J Biomed Opt. 2022 Jul;27(7):076005. doi: 10.1117/1.JBO.27.7.076005. J Biomed Opt. 2022. PMID: 35864574 Free PMC article.
-
Comprehensive intravascular imaging of atherosclerotic plaque in vivo using optical coherence tomography and fluorescence lifetime imaging.Sci Rep. 2018 Sep 28;8(1):14561. doi: 10.1038/s41598-018-32951-9. Sci Rep. 2018. PMID: 30267024 Free PMC article.
-
Dual-modality optical coherence tomography and frequency-domain fluorescence lifetime imaging microscope system for intravascular imaging.J Biomed Opt. 2020 Sep;25(9):096010. doi: 10.1117/1.JBO.25.9.096010. J Biomed Opt. 2020. PMID: 33000570 Free PMC article.
-
Intravascular Polarimetry: Clinical Translation and Future Applications of Catheter-Based Polarization Sensitive Optical Frequency Domain Imaging.Front Cardiovasc Med. 2020 Aug 28;7:146. doi: 10.3389/fcvm.2020.00146. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33005632 Free PMC article. Review.
-
Multi-modality intravascular imaging for guiding coronary intervention and assessing coronary atheroma: the Novasight Hybrid IVUS-OCT system.Minerva Cardiol Angiol. 2021 Dec;69(6):655-670. doi: 10.23736/S2724-5683.21.05532-0. Epub 2021 Mar 11. Minerva Cardiol Angiol. 2021. PMID: 33703857 Review.
Cited by
-
High-speed, long-range and wide-field OCT for in vivo 3D imaging of the oral cavity achieved by a 600 kHz swept source laser.Biomed Opt Express. 2024 Jun 27;15(7):4365-4380. doi: 10.1364/BOE.528287. eCollection 2024 Jul 1. Biomed Opt Express. 2024. PMID: 39022551 Free PMC article.
-
Intracoronary Optical Coherence Tomography: Technological Innovations and Clinical Implications in Cardiology.Curr Treat Options Cardiovasc Med. 2025;27(1):44. doi: 10.1007/s11936-025-01103-4. Epub 2025 Jul 24. Curr Treat Options Cardiovasc Med. 2025. PMID: 40727314 Free PMC article. Review.
-
Adaptive contour-tracking to aid wide-field swept-source optical coherence tomography imaging of large objects with uneven surface topology.Biomed Opt Express. 2024 Jul 29;15(8):4891-4908. doi: 10.1364/BOE.533399. eCollection 2024 Aug 1. Biomed Opt Express. 2024. PMID: 39347000 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
