Poor outcomes for trial-ineligible patients receiving polatuzumab for relapsed/refractory diffuse large B-cell lymphoma in routine care: An Australian Lymphoma and Related Diseases Registry project
- PMID: 38633125
- PMCID: PMC11020125
- DOI: 10.1002/jha2.870
Poor outcomes for trial-ineligible patients receiving polatuzumab for relapsed/refractory diffuse large B-cell lymphoma in routine care: An Australian Lymphoma and Related Diseases Registry project
Abstract
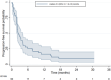
Polatuzumab vedotin (Pola) is an approved therapy in combination with rituximab and bendamustine for relapsed or refractory diffuse large B-cell lymphoma (RR-DLBCL) based on positive results of the landmark phase II randomised G029365 trial. However, trial results for many approved novel therapies in RR-DLBCL have not been replicated in routine care cohorts, as RR-DLBCL patient populations are heterogeneous and trial eligibility is increasingly restrictive. We evaluated outcomes from pola ± bendamustine and rituximab in patients with RR-DLBCL enrolled in a compassionate access program with no alternative treatment options identified via the Australasian Lymphoma and Related Diseases Registry according to their eligibility for the original phase II published study. Of 58 eligible patients, 74% met the criteria deeming them ineligible for the G029365 original study at the time of pola's commencement. Median progression-free survival and overall survival in our cohort were 2.3 and 3.5 months, respectively. In contrast to the landmark trial cohort, more of our patients ceased therapy prior to completion, the majority due to progressive disease and only 8/58 received any subsequent treatment. Dismal outcomes in this Australian real-world population demonstrate trial eligibility is challenging to meet, and newer treatments can be difficult to deliver in routine care. Clinically applicable results from therapeutic studies require trial cohorts to reflect representative clinical populations wherever possible, and more research is required to address the benefit of novel agents in the increasing majority who are ineligible for modern studies.
Keywords: antibody‐drug conjugates; diffuse large B‐cell lymphoma; immunotherapy; polatuzumab vedotin; relapse; trial eligibility.
© 2024 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Briony Shaw, Eliza Chung, Edward Yoo, Rory Bennett, Callum Birks, Jock Simpson, Sumita Ratnasingam, Tasman Armytage, Denise Lee, Colm Keane, Stephanie Wallwork all declare no conflicts of interest. Anna Johnston: Consulting or advisory role, honoraria: Merck Sharpe & Dohme, Roche, Link, BeiGene, Sanofi, EUSA Pharma, Novartis, Chan Y Cheah: Consulting or advisory role, honoraria: Roche, Janssen, Gilead, AstraZenecca, Lilly, TG therapeutics, BeiGene, Novartis, Menarini, Dizal, AbbVie, Genmab, Bristol Myers Quibb; Research funding: Bristol Myers Quibb, Roche, AbbVie, Merck Sharpe & Dohme, Lilly, Nada Hamad: Honoraria: Roche, Janssen, Gilead, AstraZenecca, BeiGene, Novartis, AbbVie, Genmab, Bristol Myers Quibb, Takeda, Jazz Pharmaceuticals, Incyte, Pfizer, Mallinckrodt Pharmaceuticals, Terumo, Allison Barraclough: Honoraria: Roche, Gilead, Novartis, BeiGene, Matthew Ku: Roche, Antengene, Genor BioPharma, Nicholas Viiala: Honoraria and advisory board: Novartis, Tara Cochrane: Consulting or advisory role: Janssen; Honoraria: Celgene (in 2019); Research funding: BeiGene, Geoffrey Chong: Research funding: Bristol Myers Quibb, Merck, AstraZeneca, Pharmacyclics, Regeneron, Hutchmed, Bayer, Incyte, Amgen, Kate Manos: Advisory/honoraria: AbbVie; Research funding: Roche, Stephen Opat: Honoraria: AbbVie, BeiGene, AstraZeneca, Bristol Myers Quibb, CSL Behring, Gilead, Janssen, Merck, Roche, Takeda; Research funding: AbbVie, BeiGene, AstraZeneca, CSL Behring, Gilead, Janssen, Merck, PharmaCytics, Roche, Takeda, Eliza A. Hawkes: Consulting or advisory role: Roche, Merck Sharpe & Dohme, AstraZeneca, Gilead, Antengene, Novartis, Regeneron, Janssen, Specialised Therapeutics; Research funding: Roche, Bristol Myers Squibb, Merck KGaA, AstraZeneca, Merck
Figures
References
-
- Hedstrom G, Hagberg O, Jerkeman M, Enblad G, Swedish Lymphoma Study G . The impact of age on survival of diffuse large B‐cell lymphoma—a population‐based study. Acta Oncol. 2015;54(6):916–923. - PubMed
-
- Liu Y, Barta SK. Diffuse large B‐cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–616. - PubMed
-
- Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales MA, Ghobadi A, et al. Survival with axicabtagene ciloleucel in large B‐cell lymphoma. N Engl J Med. 2023;389(2):148–157. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous